Chlamydia build their own entrance into human cells
Advertisement
Chlamydia, a type of pathogenic bacteria, need to penetrate human cells in order to multiply. Researchers from Heinrich Heine University Düsseldorf (HHU) have now identified the bacterial protein SemC, which is secreted into the cell and restructures the cell membrane at the entry site. SemC forces the cell’s own protein SNX9 to assist it in this process. Together with scientists from Paris and Munich, a team of researchers working under Prof. Dr. Johannes Hegemann and Dr. Katja Mölleken has published these findings in the journal Proceedings of the National Academy of Sciences of the United States of America (PNAS).
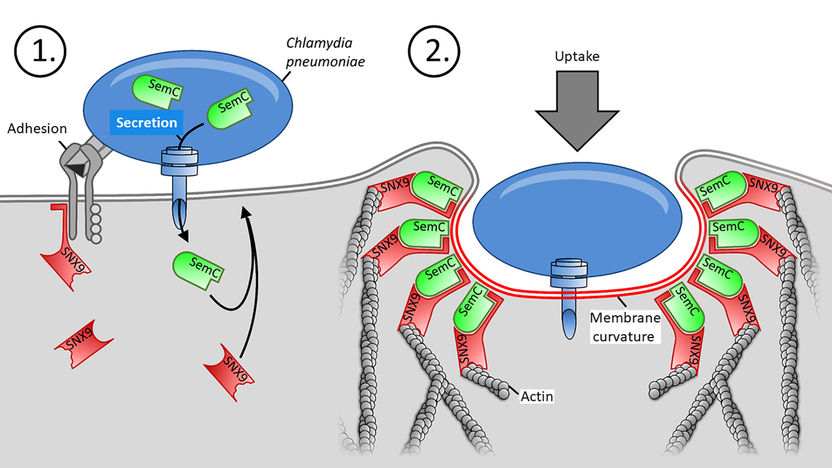
With help of adhesins (grey), a Chlamydium binds to the cell and secretes its SemC (green). It bends the PM, there binds the human SNX9 (red) and allows the bacteria to enter the cell there.
HHU / Dr. Sebastian Hänsch
There are two types of Chlamydia that infect humans: Chlamydia trachomatis and Chlamydia pneumoniae (Cpn). The former trigger sexual diseases, while Cpn lead to acute infections of the upper and lower respiratory tract. Cpn are also linked to various chronic diseases such as bronchitis and asthma as well as to lung cancer, Alzheimer’s disease and atherosclerosis. The majority of the German population will be infected with these bacteria over the course of their lives.
In order to invade the human cell, the Chlamydia must first penetrate the human cell’s membrane, known as the ‘plasma membrane’ (PM). The membrane comprises a lipid bilayer with proteins stored inside. By indenting parts of the PM, the cell can absorb fluid and particles from its environment into the cell interior, a process referred to as ‘endocytosis’.
Pathogens such as Chlamydia, which also need to get inside the cell, hijack the endocytosis mechanism for their own ends. Prof. Dr. Johannes Hegemann’s working group at the HHU Institute of Functional Microbial Genomics has now identified a chlamydial protein that plays the decisive role in penetration of Cpn into the human cell. The protein is named SemC, and it was discovered by Dr. Gido Murra from Prof. Hegemann’s working group.
In a first step, the Chlamydium succeeds in secreting SemC into the host cell. The fellow researchers from the Pasteur Institute in Paris were able to demonstrate that SemC is transported directly by the bacterium into the cell interior using a mechanism employed by many pathogenic bacteria that resembles a ‘protein needle’. Once inside the cell, the protein binds to the inner side of the PM and changes its structure locally. This changes the design of the membrane, bending it more than normal. Dr. Katja Mölleken has this to say: “With SemC, we have discovered the very first protein of an infectious agent that is able to change the PM in this way.”
The more pronounced membrane curvature triggered by SemC then causes the body’s own protein SNX9 to bind to this site, where it binds both to the curved membrane and to the SemC waiting there, thus amplifying the curvature even more. The SNX9 protein is essential for the endocytosis processes in human cells, as it builds up the actin cytoskeleton at the indented PM. The binding of SNX9 to the PM, caused by SemC, then allows the Chlamydium to penetrate the cell from the outside through a process of endocytosis at the curved part of the PM and to continue to multiply inside the cell. “The structure of the PM of the host cell is therefore an important factor in facilitating the infection of a cell by the pathogen,” emphasises Dr. Sebastian Hänsch. And Dominik Spona adds: “In this way, the bacterium basically creates its own door into the cell.”
The research group has found further important evidence of the interaction between SNX9 and SemC induced by Cpn. The scientists at the German Center for Neurodegenerative Diseases in Munich created human cells in which the quantity of SNX9 protein had been heavily reduced. Dominik Spona explains: “In these cells, it was much more difficult for Cpn to use endocytosis to penetrate the plasma membrane and to infect the cell.”
The discovery opens up new possibilities for treating chlamydial infections and for developing targeted vaccines to fight off the bacteria at an early stage. Head of the working group, Prof. Hegemann, says: “Once the precise mechanism has been decoded, potential points of attack can be identified for blocking this mechanism, for example by inhibiting the binding of SemC to the PM or to the body’s own SNX9 protein.”
Original publication
Sebastian Hänsch, Dominik Spona, Gido Murra, Karl Köhrer, Agathe Subtil, Ana Rita Furtado, Stephan F. Lichtenthaler, Bastian Dislich, Katja Mölleken, and Johannes H. Hegemann; "Chlamydia-induced curvature of the host-cell plasma membrane is required for infection"; Proc Natl Acad Sci U S A. 2020 Jan 21.