Modified tuberculosis vaccine as a therapy for cancer of the bladder
The human immune system can recognize and eliminate not only germs but also cancer cells. This is why treatments with weakened germs can help the immune system in its fight against cancer. Researchers at the Max Planck Institute for Infection Biology in Berlin have genetically modified the tuberculosis vaccine BCG in a way that it stimulates the immune system more specifically. Consequently, the new vaccine offers much greater protection against tuberculosis. A clinical study with patients suffering from cancer of the bladder has now shown that a therapy with VPM1002 could successfully prevent the recurrence of tumours in almost half of the patients who had not responded previously to the BCG therapy. The results could lead to the early approval of the drug for the treatment of cancer of the bladder so that as many patients as possible can profit from this quickly.
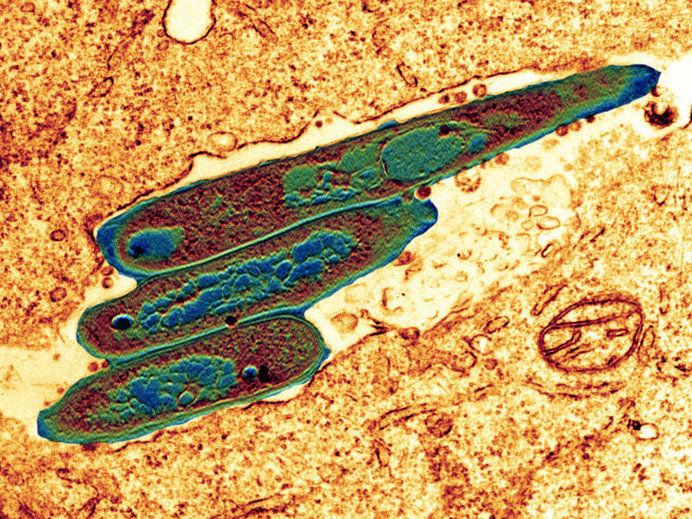
Bacteria of the weakened tuberculosis vaccine strain (BCG) inside a macrophage, a phagocyte of the immune system.
© MPI für Infektionsbiologie - CF Microscopy / Volker Brinkmann
Already at the end of the 19th century, doctors observed that the tumour in some cancer patients shrank if the patients suffered from a bacterial infection with a high fever. These findings sparked interest in immunotherapy for cancer. Immunomodulatory treatments can specifically stimulate the immune system. As a result, the body’s own immune system is supported in its fight against the tumour, leading to a reduction in the size of the tumour.
The tuberculosis vaccine Bacille Calmette-Guérin (BCG) that was introduced back in the 1920ies contains weakened pathogens of bovine tuberculosis, which can also be transmitted to humans. Tests in the 1970ies showed that BCG is also effective against bladder cancer, one of the most common tumour diseases in Europe.
Bladder treatment with weakened germ
Immunotherapies for solid tumours often have little success, though the use of BCG for bladder tumours developed into a standard therapy. During this treatment, the bladder is repeatedly flushed with the weakened germ over a period of six weeks. This triggers an immune response that, although not aimed specifically at the tumour, does presumably activate the body’s own killer cells that in turn recognize and specifically kill the altered tumour cells.
Nevertheless, the share of patients who have managed to fully overcome cancer after the BCG therapy is low. Furthermore, flushing with BCG has serious side-effects such as fever, incontinence or flu-like symptoms, so that many patients discontinue the therapy prematurely. However, the tumour returns in 30 to 40 percent of such treated patients. In these cases, the bladder has to be removed completely, though highly unwanted, but as an ultimate resort.
Vaccine development
Stefan Kaufmann from the Max Planck Institute for Infection Biology in Berlin has developed the BCG vaccine further with his colleagues. The researchers modified the weakened tuberculosis bacteria in a way that it can be recognized better by the immune system. “The new vaccine VPM1002 is absorbed like BCG by the immune system’s phagocytes, that can then subsequently identify their targets – tuberculosis bacteria and cancer cells – much better,” explains Kaufmann. Improved protection against infection with tuberculosis bacteria has already been proven using VPM1002.
VPM1002 has been developed by the world’s biggest vaccine manufacturer, the Serum Institute of India in collaboration with the Vakzine Projekt Management GmbH (VPM) from Hannover. A clinical study (SAKK 06/14) under the direction of Cyrill A. Rentsch, University Hospital Basel, Switzerland, together with the Swiss Group for Clinical Cancer Research (SAKK) are now investigating whether it is possible to avoid removing bladders from patients suffering from cancer of the bladder by using VPM1002. A Phase I study showed that the new vaccine is safe and well tolerated.
Patients suffering from cancer of the bladder, where the cancer had returned after removal of the tumour and a subsequent standard BCG therapy, were then treated in a Phase II study. “Over 49 percent of the patients treated with VPM1002 were free from tumours in the bladder after 60 weeks,” says Leander Grode, who developed VPM1002 together with Stefan Kaufmann and is now Managing Director of VPM. Tumour-free patients avoid a removal of the bladder.
Quick approval
The results have encouraged the developers to apply for early regulatory approval as soon as possible. As a result, patients suffering from cancer of the bladder who no longer respond to conventional therapies, can profit from the new drug as quickly as possible and therefore, if responding to VPM1002 therapy, avoid a removal of the bladder. Talks are now planned with the European Medicines Agency to bring about its Europe-wide approval as quickly as possible.
The Serum Institute of India is a strong partner that can produce the required amounts of medicines swiftly and whose strategy also delves deeper into cancer therapy. “I am extremely pleased with the results of VPM1002 in bladder cancer therapy as we will be able to provide high volume and consistent supply of such an important product with unique method of manufacturing for addressing unmet need in the field of bladder cancer to the entire world”. says Adar C. Poonawalla, the CEO of SIIPL.
The technology on which the vaccine VPM1002 is based was licensed from Max-Planck-Innovation, the technology transfer organization of the Max Planck Society. “We are delighted to have found a suitable licensee in VPM to develop the results of the Max Planck Society’s research work further. Although VPM was not an established vaccine company at the time, it managed to cope with typical development obstacles and set-backs and demonstrated the efficacy and safety of the bladder cancer vaccine. We hope that patients will soon be able to be treated effectively following these results,” says Dieter Link, Patent- and Licensing Manager at Max-Planck-Innovation.