Intestinal bacteria could influence the development of multiple sclerosis
A tryptophan-free diet in mice changes the composition of the intestinal bacteria and protects against symptoms of experimentally generated multiple sclerosis.

In mice with MS symptoms, the spinal cord is severely inflamed. This becomes clear under the microscope, through the detection of specialized inflammatory cells (image on the left, in dark brown and marked with arrows). A tryptophan-free diet prevents this infiltration of inflammatory cells into the spinal cord (image on the right).
Copyright: Melanie Keil / DKFZ
Tryptophan is an essential amino acid, i.e. a protein component which cannot be produced by the body itself, but must be obtained from the diet. Metabolic products of tryptophan serve as chemical messengers for numerous important bodily functions. They control specific immune cells, for example, or help to strengthen the intestinal barrier. A diet which specifically omits tryptophan alters the composition of the intestinal bacteria in mice, and surprisingly results in the animals not developing symptoms of multiple sclerosis (MS). This has been demonstrated by scientists from the Cluster of Excellence "Precision Medicine in Chronic Inflammation" at the Institute of Clinical Molecular Biology (IKMB) at Kiel University (CAU), in cooperation with the German Cancer Research Center (Deutsches Krebsforschungszentrum, DKFZ) in Heidelberg.
Multiple sclerosis (MS) is a chronic inflammatory neurodegenerative disease of the central nervous system. With MS, the own immune system attacks the insulation of nerve fibers in the central nervous system, and gradually destroys them. This disturbs the transmission of signals in the nerves. Over time, these changes can disrupt the motor functions or sensory perception of those affected. Previous studies suggested that a combination of genetic predisposition and environmental factors causes the disease.
The research by Dr. Maren Falk-Paulsen and Professor Philip Rosenstiel from the Institute of Clinical Molecular Biology (IKMB) at Kiel University (CAU), in cooperation with a team led by Professor Michael Platten from the DKFZ in Heidelberg, shows that the intestinal microbiome, i.e. the totality of the bacteria in the gut, could also play an important role. They used a mouse model of multiple sclerosis, in which the body's own immune cells attack a specific envelope protein of the nerve insulation in the central nervous system, and thereby cause typical MSsymptoms. However, mice that received a special diet in which the amino acid tryptophan was missing, did not develop any MS symptoms in this model. In these mice, the aggressive immune cells did not enter the spinal cord. This protective effect was dependent on the presence of certain bacteria in the gut - if these were missing, then the protection against MS also disappeared.
"By omitting the amino acid tryptophan, the composition of the intestinal bacteria changes, which sends a so far unknown signal to the immune cells. We do not yet know which mechanisms are behind this phenomenon. We want to examine this more closely in future," said Dr. Maren Falk-Paulsen, researcher at the IKMB and member of the Cluster of Excellence PMI.
Scientists from the Cluster of Excellence PMI at the IKMB led by Professor Philip Rosenstiel have already been researching how tryptophan influences the intestinal microbiome and chronic inflammation for a long time. "In previous work, we have been able to show how tryptophan affects inflammations in the gut," explained Professor Rosenstiel, director at the IKMB and board member of the Cluster of Excellence PMI. "Now we have shown that the omission of tryptophan also affects inflammatory reactions in other parts of the body, and this via a mechanism independent of the previously known signaling pathways. Based on our results, we hope to find a new target for treating MS in future," added Rosenstiel.
Original publication
Most read news
Original publication
Jana K. Sonner*, Melanie Keil*, Maren Falk-Paulsen*, …, Philip Rosenstiel & Michael Platten; "Dietary tryptophan links encephalogenicity of autoreactive T cells with gut microbial ecology"; Nature communication; 2019; (*the authors contributed equally to the publication)
Topics
Organizations
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents

Rapid genome analysis of a Whippet sighthound sets new standard for biodiversity research - “We are demonstrating that we can obtain and analyse complete genome information in a matter of days – not months”
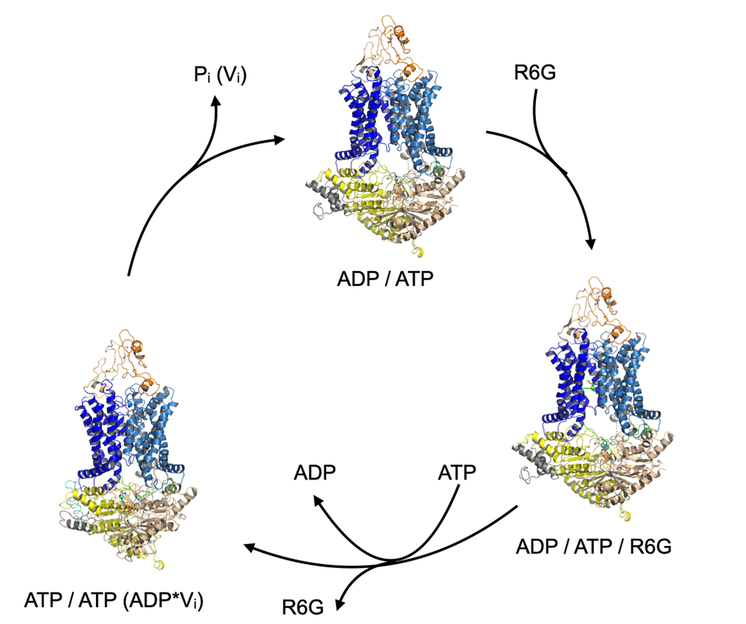
How resistant germs transport toxins at molecular level - Results could help develop mechanisms to combat dangerous pathogens
Pharmaceutical_policy
Evotec achieves clinical development milestone as part of its multi-target alliance with Bayer
Synaptic_pharmacology
New, carbon-nanotube tool for ultra-sensitive virus detection and identification

Genome sequence of the morelle decoded - Research team uses novel technologies to investigate the complex genetic material of the sour cherry variety and draw conclusions about the origin of the fruit species
"Waltzing" nanoparticles could advance search for better drug delivery methods - "Swirling and spinning" of therapeutic nanoparticles

BRAIN takes over anti-bitter patents and utility patent portfolio from BASF






















































