EMBL spins the Sleeping Beauty transposase
EMBL scientists have developed a new variant of the Sleeping Beauty transposase. It has dramatically improved biochemical features, including enhanced stability and intrinsic cell penetrating properties. This transposase can be used for genome engineering of stem cells and therapeutic T cells. As such it is extremely valuable for use in regenerative medicine and cancer immunotherapy. The underlying genome engineering procedures will in the future also reduce costs and improve the safety of genome modifications.

EMBL spins the Sleeping Beauty transposase
Scienseed
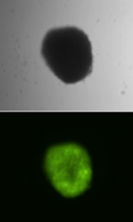
The team, comprising researchers from the European Molecular Biology Laboratory, the Universitätsklinikum Würzburg and the Paul-Ehrlich-Institut, managed to design a new variant of the Sleeping Beauty transposase with dramatically improved biochemical properties, enabling the direct use of the transposase protein for genome modifications. “The protein we developed can be delivered into mammalian cells and remains fully functional, enabling efficient and stable genome modifications in target cells on demand,” explains Orsolya Barabas, group leader at EMBL Heidelberg.
The delivery and efficient genetic engineering can be used on different types of cells, including human stem cells and T lymphocytes. The latter can be genetically modified to produce an artificial chimeric antigen receptor (CAR) for use in cancer immunotherapy. The new type of Sleeping Beauty transposase developed by the researchers not only enables direct protein delivery, but also penetrates cells autonomously. The latter feature was not planned for the new variant and was only discovered when it was studied in action. This was a pleasant surprise, as it is the first of its kind with this characteristic. “All these features open new avenues for CAR-T cell production and other gene therapies,” explains Irma Querques, PhD student at EMBL and a lead author of the paper. As such, this is a breakthrough compared to other existing variants of the Sleeping Beauty transposase.
Sleeping Beauty
The Sleeping Beauty transposon system consists of a transposase and a transposon to insert specific sequences of DNA into the genomes of animals.
The transposase can be encoded either within the transposon or can be supplied by another source, in which case the transposon becomes a non-autonomous element. Non-autonomous transposons are most useful as genetic tools, because after insertion they cannot independently continue to excise and re-insert themselves. All of the DNA transposons identified in the human genome and other mammalian genomes are non-autonomous because, even though they contain transposase genes, these genes are non-functional and unable to generate a transposase that can mobilise the transposon.
The Sleeping Beauty transposase was resurrected from inactive copies in fish genomes by Zoltan Ivics and his colleagues in 1997, creating the first transposon tool that worked efficiently in vertebrate cells. Since then it has been used for many applications in genetics, including gene therapy.
A direct application
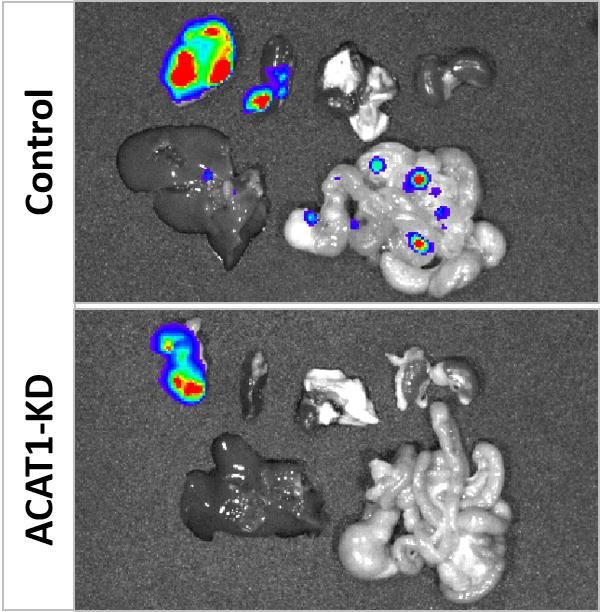
While EMBL researchers generally focus on fundamental research, these results lead to a direct medical application. “The new transposase and the genome engineering procedures we developed will find direct use in therapeutic cell engineering,” highlights Michael Hudecek from the Universitätsklinikum Würzburg the importance of the results. “Already in this first study, we demonstrate the utility of our method for CAR-T cell production and its efficacy in a mouse model.” Now Hudecek and his colleagues will continue research with the transposase for use in human patients.
“Our method further offers attractive use in stem cell engineering and I am sure it will find its applications in regenerative medicine and associated research. One of the most outstanding advantages of the novel technology is that it enables industrial-scale, pharmaceutical production of the transposase, making the Sleeping Beauty gene delivery system even more attractive for companies for future therapeutic applications,” explains Zoltán Ivics, from the Paul-Ehrlich-Institut.
The design principles of the transposase and protocols developed by the EMBL group will also help to create similar strategies for other transposon systems. The team is curious to further explore the mechanisms behind the cell penetrating property of the Sleeping Beauty transposase and whether these mechanisms can be transferred to other proteins as well. “The availability of our new Sleeping Beauty variant will also facilitate research towards understanding its molecular mechanisms, which in turn will promote the rational design of more advanced transposon tools,” adds Cecilia Zuliani from EMBL; another lead author of the paper.
While this will require further work, Barabas highlights one immediate impact: “For now, our new cell engineering procedure will lead to reduced costs and – through improved fidelity and control of the method – improved safety of medically relevant genome modifications.”
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Gene therapy
Genetic diseases once considered untreatable are now at the center of innovative therapeutic approaches. Research and development of gene therapies in biotech and pharma aim to directly correct or replace defective or missing genes to combat disease at the molecular level. This revolutionary approach promises not only to treat symptoms, but to eliminate the cause of the disease itself.

Topic world Gene therapy
Genetic diseases once considered untreatable are now at the center of innovative therapeutic approaches. Research and development of gene therapies in biotech and pharma aim to directly correct or replace defective or missing genes to combat disease at the molecular level. This revolutionary approach promises not only to treat symptoms, but to eliminate the cause of the disease itself.