DNA repair: Opening the hatch to heal the break
LMU researchers have determined the structure of a key enzyme complex that is involved in DNA repair, and traced the cycle of conformational changes that it undergoes while performing its biochemical function.
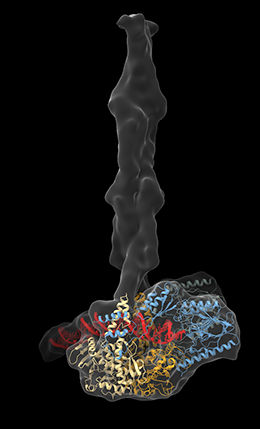
MR Complex.
L. Käshammer
Various types of DNA damage can have serious repercussions, both for the individual cells in which they occur and for the organism as a whole. Instances of simultaneous breakage of both strands of the DNA helix are particularly harmful. Such double-strand breaks (DSBs) can be induced by radiation, as well as by environmental toxins, and can lead to cell death. However, cells possess efficient mechanisms for detecting and repairing DSBs. A molecular machine known as the MR complex plays a central role in this process. It recognizes and binds to DSBs, and initiates repair of the broken double helix. A team of scientists led by Professor Karl-Peter Hopfner, who holds the Chair of Structural Molecular Biology at LMU’s Gene Center, has now deciphered the complete three-dimensional structure of the MR complex and determined how it works.
Hopfner’s group had previously shown that the MR complex is made up of four proteins. Two of these are nucleases, which themselves cut DNA strands. The others are ATPases – enzymes that remove a phosphate group from the molecule ATP, and in so doing release chemical energy. The complex itself has an open architecture. But when it encounters a DSB, it binds to the DNA and brings the ends together, acting as the molecular equivalent of a hook-and-loop fastener. The complex then detaches any chemical adducts that block the ends of the break and removes further subunits from the DNA. These operations then provide a ‘clean break’ in preparation for the repair process itself. “Because of the intricate architecture of the complex, the conformational changes involved in this process had not been elucidated,” says Lisa Käshammer, lead author of the paper. The ATPase enzymes contain prominent filamentous structural elements that are referred to as coiled-coils, which are made up of closely apposed and helically ordered amino-acid sequences. These filaments are long and flexible, and their mobility had made it impossible to discern their precise courses using conventional X-ray crystallographic methods.
The team turned to cryo-electron microscopy to get around this problem, and succeeded in determining the complete structure of the MR complex found in the bacterium Escherichia coli. The bacterial complex is somewhat less complicated than its counterpart in higher organisms, but in their overall structure the two forms are similar to each other. The new structure now makes it possible to discern how the nucleases actually bind to the DNA – a question that had remained open up to now. “In the previously published structures, the DNA was either positioned relatively far from the active center of the nuclease, or bound in such a way that the active center was inaccessible,” Käshammer explains. The new structure reveals that binding to the DNA causes the nuclease to execute a 120-degree turn, which opens up a channel that enables the DNA to bind to the active center of the enzyme.
The study also clarifies the function of the coiled-coil regions. As the new structural model demonstrates, these segments form a brace that stabilizes the DNA, and are actively involved in the binding and processing of the DNA ends. “Our findings constitute an important advance in the quest for a deeper understanding of the complex mechanisms that make it possible for these enzymes to repair double-strand breaks in DNA,” says Hopfner. New insights into the function of the complex are also of potential medical significance, as DSBs play a significant role in the pathogenesis of many types of cancer.