Plan B for Cholesterol Transport
Research team proves the existence of a previously unknown alternative cholesterol transport mechanism inside cells
Advertisement
cholesterol is a vital cell building block in humans and animals, and an integral part of the so-called cell membrane. This boundary layer separates the interior of the cell from the neighbouring cells and the surrounding environment. By means of certain proteins, it ensures that various chemical messengers can enter or exit the cell. In addition, cholesterol is the precursor of various hormones and bile acids. The level of cholesterol in the blood is also associated with numerous diseases, in particular of the cardiovascular system, but also with the very rare but particularly serious so-called lysosomal storage disorders. For many years, researchers at the Institute of Biochemistry at Kiel University (CAU) have been investigating areas such as the intracellular transport of cholesterol, and which processes and proteins are involved in this. Thus, in 2013 they succeeded in characterising a protein which is centrally involved in these processes, the so-called LIMP-2 protein, and described its molecular blueprint. The resulting spatial model of the protein surprisingly revealed a tunnel structure, which in line with other proteins indicated a possible transport function of LIMP-2. In research work conducted jointly with international colleagues, including from Zhejiang University and the University of Helsinki, the Kiel research team has now proven the involvement of the LIMP-2 protein in cholesterol export from lysosomes, the cell organelles which break down many kinds of biomolecules. Thus, the researchers described a previously unknown method of cholesterol reduction inside cells.
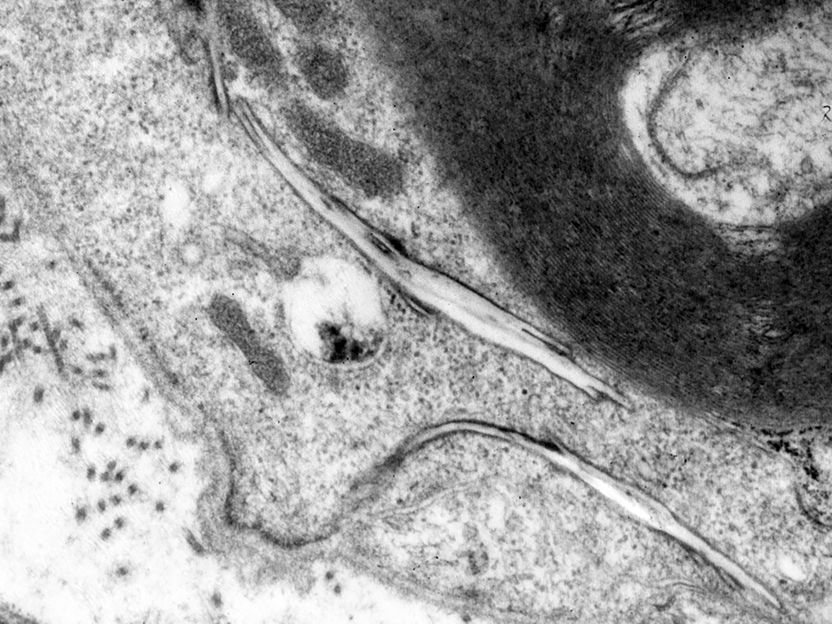
An electron microscope image showing how cholesterol accumulates in a crystalline-like shape (centre) in certain nerve tissues when transport processes are disrupted.
© Prof. Renate Lüllmann-Rauch
Distribution nodes of the cell
The key focus of the Kiel biochemistry research team led by Professor Paul Saftig is the role of lysosomes. These organelles in human and animal cells are responsible for the transport as well as the conversion and degradation of various substances, including cholesterol. It was already known that a particular protein is responsible for the transport of cholesterol from the lysosomes. "The striking tunnel structure of our newly characterised protein grabbed our attention," said biochemist Saftig regarding the starting point of the current research. "Through our experiments, we have now been able to confirm that LIMP-2 is actually responsible for an alternative mechanism of cholesterol transport," continued Saftig.
Why this parallel mechanism exists, which also operates less efficiently than the already known mechanism, remains unclear. "We have recently determined that both pathways can to some extent take over each other’s function," emphasised Saskia Heybrock, a doctoral researcher at the Institute of Biochemistry and one of the lead authors of the publication. "If we disable the LIMP-2 transport mechanism, there is not a significant build-up of cholesterol, because it is still removed via the primary pathway," said Heybrock. Nevertheless, the researchers suspect that the two pathways are not entirely redundant, and that the LIMP-2 tunnel could be particularly responsible for certain cell types.
Harmful cholesterol build-up
The international research team obtained evidence for the function of the LIMP-2 protein tunnel using various methods. With light microscopic analysis, the researchers were able to essentially catch the cholesterol 'red-handed': thanks to a fluorescent marking, it could be seen leaving the tunnel. In addition, electron microscope images even showed how cholesterol accumulates in certain nerve tissues as soon as the LIMP-2 pathway is not available. A functional gene analysis also revealed that the deactivation of the genetic information responsible for the LIMP-2 protein led to a disruption of the cellular cholesterol balance. In this case, the cells increasingly activate signals which indicate a cholesterol deficit that could lead to a problematic accumulation of cholesterol, which may be involved in the pathogenesis.
“In our new work, we were able to describe an additional pathway which is involved in cholesterol regulation," said Saftig regarding the importance of the new publication. "The increasingly precise understanding of the cellular transport and distribution processes will help us in future to find starting points for new treatments," continued Saftig. Therefore, the scientists hope in particular to be able to develop drugs to combat the lysosomal storage disorders. In a follow-up step towards this goal, the Kiel researchers initially want to clarify all the different functions of the seemingly redundant pathways in the intracellular transport of cholesterol.

























































