Exploring genetic 'dark matter,' researchers gain new insights into autism and stroke
With its elegant double helix and voluminous genetic script, DNA has become the of darling of nucleic acids. Yet, it is not all powerful. In order for DNA to realize its potential--for genes to become proteins--it must first be transcribed into RNA, a delicate molecule that requires intense care and guidance.
"Gene expression is a lot more complicated than turning on a switch," says Robert B. Darnell, the Robert and Harriet Heilbrunn Professor. "There's a whole layer of regulation that alters both the quality and quantity of a protein that's produced from a gene. And much of it happens at the level of RNA."
In the brain, RNA's job as a gene tuner is vital to ensuring that the right proteins are made at the right time; and when this process go awry, the consequences can be serious. Darnell's lab recently found that the brain's response to stroke depends on the precise regulation of a subtype of RNA; and they have also learned that mutations affecting gene regulation underlie some cases of autism spectrum disorder.
Genome's little helper
Whereas DNA is stuck inside a cell's nucleus, RNA is fairly mobile. In the brain, so-called messenger RNAs can be found at the connections between neurons, called synapses, where they are translated into proteins that affect brain signaling. This process is regulated by another class of RNAs, known as miroRNAs, which can rapidly promote or suppress protein production in response to dynamic changes in the brain.
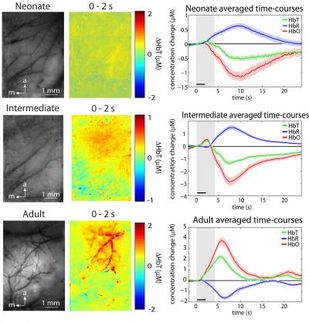
In a recent experiment described in Cell Reports, Darnell and his colleagues tracked microRNA activity in the mouse brain following a simulated stroke. Using a technique called crosslinking immunoprecipitation, or CLIP, they found that stroke prompts a dramatic reduction in a subset of microRNAs known as miR-29s. Typically, these molecules limit the production of two proteins called GLT-1 and aquaporin; and when miR-29 levels drop, the researchers found, these proteins are produced in higher-than-usual quantities.
GLT-1 is responsible for getting rid of extra glutamate, a chemical that is produced in abundance during stroke and can harm the brain if left unchecked. An uptick in production of this protein therefore seems to mitigate stroke-associated brain damage. Increased aquaporin, on the other hand, exacerbates tissue swelling, further threatening an already-imperiled brain. In short, a drop in miR-29s appears to simultaneously help and hinder stroke recovery. The good news is that a better understanding of how both of these processes work might guide the development of new and very precise medical tools.
"This research suggests potential drug targets for treating stroke," says Darnell. "By artificially inducing more GLT-1 mRNA with a drug, for example, you could regulate the amount of glutamate that's getting sucked up and reduce damage to the brain."
Covert mutations
To understand what causes a person's disease, researchers often look for mutations in genes--also known as the "coding" regions of DNA--that lead to the production of dysfunctional proteins. However, this general strategy works only for diseases that run in families and are driven by specific protein irregularities, which isn't the case for some complex conditions. For example, though studies have identified many different coding mutations that contribute to the development of autism spectrum disorder (ASD) and epilepsy, together these mutations account for only about a quarter to a third of cases.
Researchers are therefore beginning to search for irregularities in the noncoding sections of DNA--regions that don't directly code for proteins, but that make RNA whose job it is to regulate genes. Once thought of as "junk DNA," these regions are now known to be critical in determining which proteins a cell makes, when it makes them, and in what quantities. And according to Darnell, analyzing noncoding DNA can be particularly useful in understanding diseases that don't adhere to conventional heredity patterns.
"Some conditions have a genetic component, but they don't come with simple family trees where you can predict the chance of a child having a disease based on the parents' genetic makeup," says Darnell. "So you need a different approach to figure out which types of mutations are underlying the disease."
To find noncoding mutations associated with ASD, Darnell and his colleagues developed a new take on the family tree. Using a large genetic database, they first analyzed the DNA of 1,790 "microfamilies," each consisting of a mother, a father, one child with ASD, and one without. They then applied a machine-learning algorithm, developed with colleagues at Princeton, to identify ways in which children with the condition were genetically different from the rest of their family members who were unaffected by the disorder.
Described in Nature Genetics, these findings suggest that by analyzing noncoding mutations, researchers may be able to better understand not only ASD but a variety of conditions, ranging from neurological disorders to heart disease.
"Noncoding DNA makes up over 98 percent of the genome, and it's largely unexplored," says Darnell. "We're showing that this genetic dark matter can fill in our understanding of diseases that coding mutations can't explain."