In the active centre of carbon dioxide conversion
Scientists reveal the features of efficient carboxylase enzymes
In order to overcome the climate crisis, two measures are required: The reduction of carbon dioxide (CO2) emissions, and removal of CO2 from the earth atmosphere. The latter is the goal of Tobias Erb's research group at the Max Planck Institute for Terrestrial Microbiology in Marburg. Their approaches not only aim to benefit climate protection, but also to secure sustainability in the long term: to filter CO2 from the air and make it usable for technology.
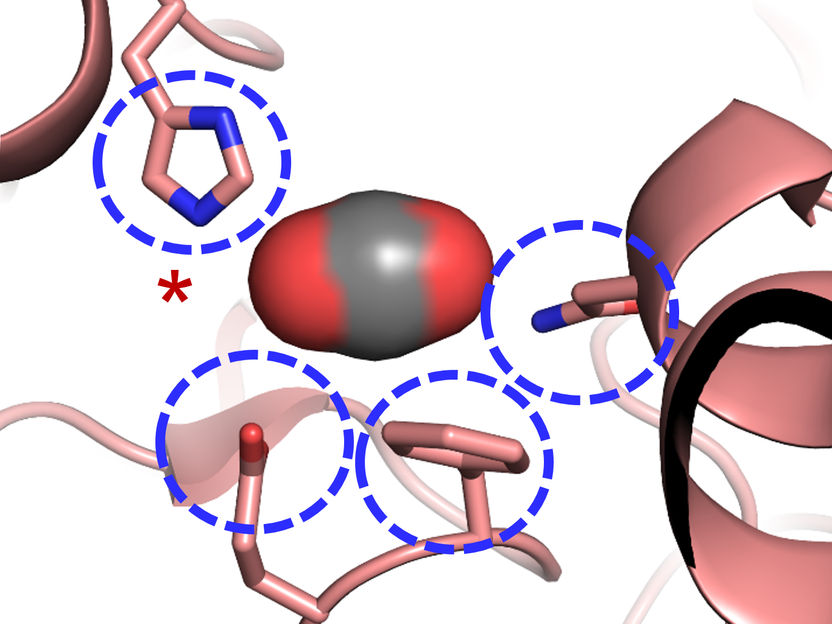
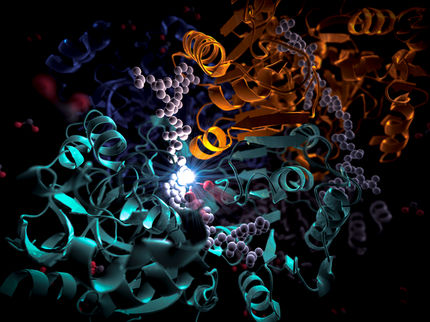
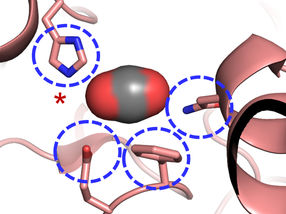
The Carboxylase active site: Four amino acids are important for CO2 binding in highly efficient CO2 fixing enzymes.
© Max-Planck-Institute for Terrestrial Microbiology/Erb
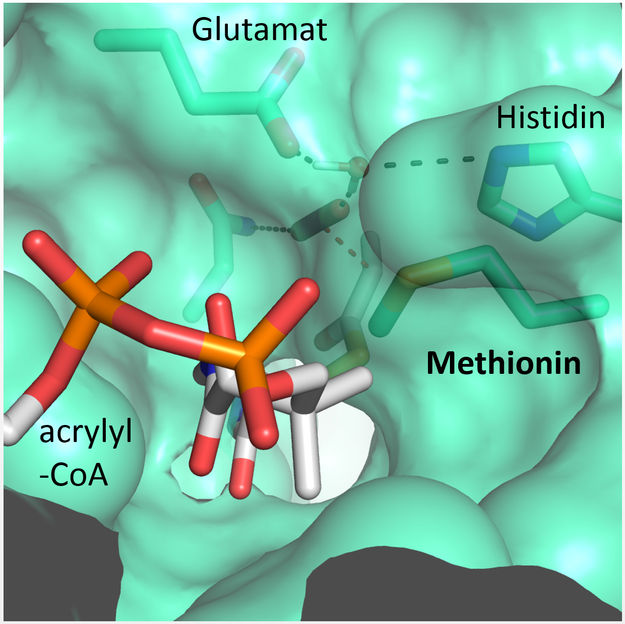
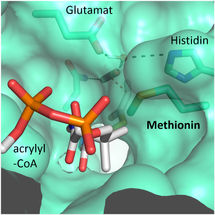
A methionine residue shields the active site from the competing water molecules.
© Max-Planck-Institute for Terrestrial Microbiology/Erb
Filtering CO2 efficiently from the air - nature can do this through photosynthesis, converting CO2 into biomass. Unlike industrial technologies which can only use the gas in a highly concentrated form (which in turn consumes fossil energy), photosynthesis works directly with ambient air containing only 0.04% gaseous carbon dioxide. Its secret lies in the enzymes, proteins that act as catalysts to mediate specific chemical reactions, such as the fixation of CO2. In photosynthesis, this reaction is driven by the enzyme RubisCO. However, the efficiency of natural photosynthesis is not very high: in more than a fourth of all cases, RubisCO metabolizes oxygen from the air, which is a strong competitor of CO2 in this reaction.
ECR enzymes are faster and more precise than RubisCo
Therefore, the Max Planck researchers in Marburg have decided on alternatives to RubisCO. Enoyl-CoA Carboxylase/Reductase enzymes (ECRs) are much more efficient than RubisCO and do not make mistakes with oxygen. After many years of scientific work in order to understand this capacity, the scientists have succeeded in building a functioning process in the test tube that fixes CO2 better than nature itself. Robustness and energy efficiency are the qualities that the team would like to bestow to their artificial photosynthesis. "Learn from the best" is the motto: nature itself serves as a model for molecular biology.
What is the reason for the high efficiency of ECRs? What is the magic spell to create a turbo CO2 fixator? Max Planck junior researchers Gabriele Stoffel and Iria Bernhardsgrütter pursued this question together with colleagues from Chile and the USA. They analyzed the ECR from the bacterium Kitasatospora setae, currently the fastest known carboxylase. Using a combined approach of structural biology, biochemistry and computer simulations, they were able to understand for the first time how the enzyme binds and converts CO2.
Teamwork in the active centre
"We were surprised to learn that only four amino acids are sufficient to provide control over the CO2 molecule”, explains Gabriele Stoffel, postdoctoral fellow in the Erb department and first author of the study. “Three amino acids – one asparagine, glutamate and a histidine – hold the CO2 in place from two sides. Another amino acid, a phenylalanine, shields the bound CO2 from water, which would inhibit the reaction”, says Stoffel.
These findings open up new paths for researchers. "We wanted to transfer the capability of binding CO2 to other enzymes. This would offer us much greater possibilities for optimizing photosynthesis," says Iria Bernhardsgrütter, PhD student in the research group. In another study, Bernhardsgrütter focused on two candidates for the protein scaffold: Propionyl-CoA synthase (PCS) and Archaeal Enoyl-CoA reductase (AER).
Enhancing CO2 fixation capacity
Both enzymes were already able to use CO2, but only with an efficiency of about five percent and with concentrated CO2. Computational models revealed that those enzymes only possessed some of the four amino acids required and those were also misaligned. Iria Bernhardsgrütter succeeded by exchanging amino acids to correct the "miscasts" in PCS. Immediately, the efficiency of CO2 increased to around 20 percent. Now the second aspect was targeted, namely shielding the binding site from water. Iria Bernhardsgrütter was also able to solve this problem: another amino acid replacement blocked the water's access to the binding site. The combination of both changes led to a carboxylation rate of almost 95%. Similar experiments with AER increased CO2-conversion efficiencies to almost 90%.
This knowledge of the exact requirements of CO2-fixing enzymes and its successful application has brought research a decisive step closer to its high goals: on the one hand, being able to filter CO2 efficiently from the atmosphere, on the other hand, integrating CO2 into sustainable use – towards the recycling of valuable substances following nature's example
Original publication
Stoffel, G. M. M.; Saez, D. A.; DeMirci, H.; Vögeli, B.; Rao, Y.; Zarzycki, J.; Yoshikuni, Y.; Wakatsuki, S.; Vöhringer-Martinez, E.; Erb, T. J.; "Four amino acids define the CO2 binding pocket of enoyl-CoA carboxylases/reductases"; Proceedings of the National Academy of Sciences 201901471 (2019).
Bernhardsgrütter, I.; Schell, K.; Peter, D. M.; Borijan, F.; Saez, D. A.; Vöhringer-Martinez, E.; Erb, T.; "Awakening the Sleeping Carboxylase Function of Enzymes:Engineering the Natural CO2‑Binding Potential of Reductases"; Journal of the American Chemical Society 141, 9778 −9782 (2019).