Stem cells moonlight to protect the stomach from bacterial invaders
A subpopulation of stem cells releases antimicrobial peptides to defend the gastric mucosa against pathogenic bacteria
Advertisement
Our mucosal surfaces are constantly exposed to numerous bacterial species, some of which can induce DNA damage in host cells. Normally this remains inconsequential, as the rapid turnover of the mucosa means damaged cells are shed within days. However, if the long-lived stem cells that continually give rise to new replacement cells receive damage it could lead to the development of cancer. Researchers at the Max Planck Institute for Infection Biology and the Charité - Universitätsmedizin in Berlin have now revealed that the gastric stem cell pool does do not merely divide to generate new daughter cells, but that they can secrete antimicrobial molecules to actively defend the stem cell niche against bacteria.
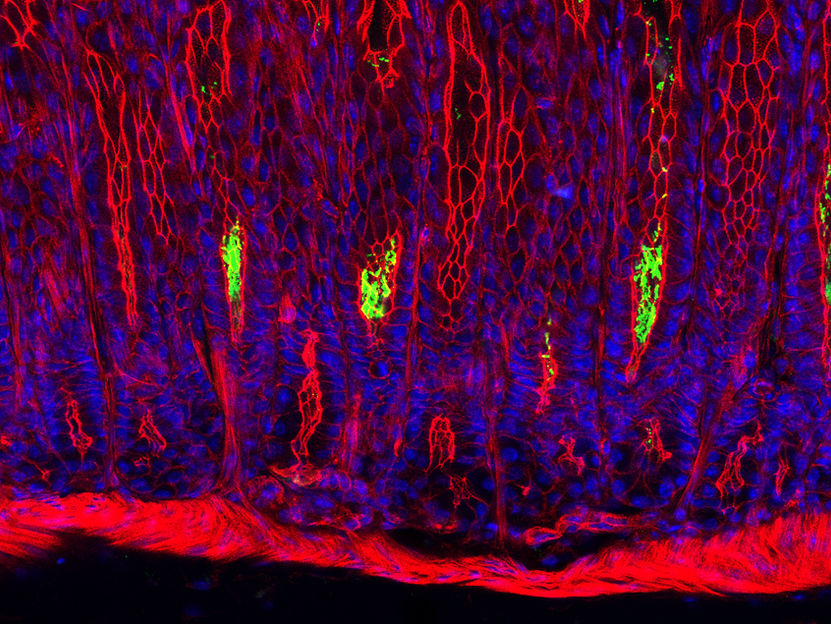
Cross-section through the gastric mucosa of a mouse: Helicobacter pylori bacteria (green) colonize the cavities of the gastric glands. The nuclei of the mucous membrane cells are depicted in blue, their cytoskeleton red.
© Sigal/ Charité
The team looked at infections with the gastric pathogen Helicobacter pylori, a bacterium which chronically colonizes the stomach of around half the world’s population, and is known to cause stomach cancer. Michael Sigal of the Charité, who led the study with the Max Planck team of Thomas F. Meyer, previously showed that the bacterium not only colonizes the surface of the gastric epithelium but is able to penetrate deep into the base of gastric glands, where the stem cells reside.
In response to the infection, the stromal cells of the underlying connective tissue produce increased amounts of a factor called R-spondin, which enhances the activity of the so-called Wnt pathway – an almost universal driver of stem cell turnover in various tissues. In response, the stem cells increase their turnover, leading to hyperplasia, a thickening of the mucosal lining that is typical for patients with gastritis.
Subpopulation of stem cells
Although the team saw that R-spondin led to a drop in the number of colonizing bacteria, they were not convinced that the increased tissue turnover alone is responsible. They now found that a subpopulation of stem cells in the very base of the gland does not respond to R-spondin with increased turnover. Instead, this population differentiates into secretory cells and begins to produce and release antimicrobial peptides into the gland – thus protecting their own niche from bacterial attack. When the team enhanced the ability of gastric stromal cells to produce R-spondin, the stem cells produced even higher amounts of antimicrobial peptides after infection and the gastric glands were completely cleared of bacteria. This demonstrates that the antibacterial compounds produced in the stem cell niche are highly effective.
According to Meyer, these findings add significant weight to our increasing awareness that the mucosal epithelium itself represents an extremely important site of immune defense. “The stem cell niche of the gastric mucosa – and likely many other tissues – has the means to protect itself from bacterial invaders. This is likely to be an important mechanism for maintaining the genetic integrity of adult stem cells”.
The findings may also have important implications for other gastrointestinal diseases, such as Morbus Crohn, which is thought to result from a failure of the normal epithelial barrier function. It is likely that here, too, the stem cell compartment has the ability to control the production of antimicrobials. “It would be of enormous clinical benefit if such antimicrobials could be used as markers to identify patients in whom this protective defense is compromised” adds Sigal. In addition, the team will continue to investigate in what way an impaired epithelial defense may play a role in carcinogenesis.
Original publication
Michael Sigal, Maria del Mar Reines, Stefanie Müllerke, Cornelius Fischer, Marta Kapałczynska, Hilmar Berger, Elvira RM Bakker, Hans-Joachim Mollenkopf, Michael E Rothenberg, Bertram Wiedenmann, Sascha Sauer, Thomas F Meyer; "R-spondin-3 induces secretory, antimicrobial Lgr5+ cells in the stomach"; Nature Cell Biology; 24 July, 2019