Making systems robust: Artificial genetic regulatory network introduced into living cell for the first time
Improving biotech and therapies
Advertisement
Both nature and technology rely on integral feedback mechanisms to ensure that systems resist external perturbations. ETH researchers have now used synthetic biology to design a new mechanism of this sort from scratch. For the first time, they have introduced it into a living cell as an artificial genetic regulatory network. This will be a useful tool for cell therapy in medicine and for biotechnology.
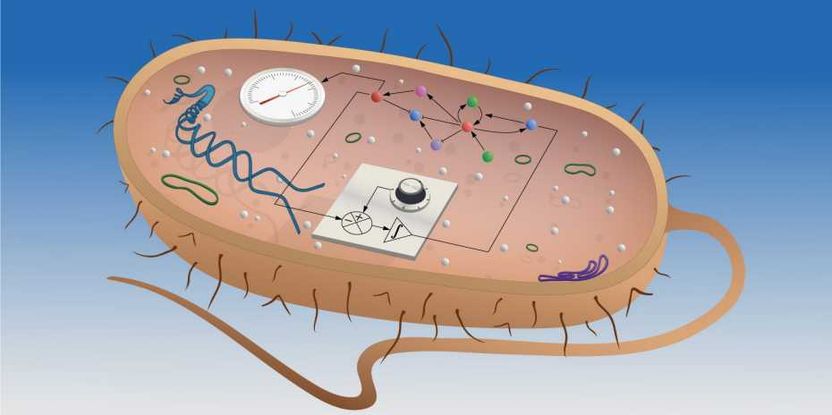
The scientists developed an integral controller (represented in front as a circuit diagram) for Escherichia coli bacteria.
ETH Zurich / Christine Khammash
The human body ensures that the calcium concentration in the blood remains constant. In the same way, an aircraft’s autopilot keeps the plane flying at a constant altitude. What they have in common is that both the body and the autopilot employ sophisticated integral feedback control mechanisms. Researchers in the Department of Biosystems Science and Engineering at ETH Zurich in Basel have now succeeded for the first time in building such an integral controller completely from scratch within a living cell, as they report in the latest issue of the journal Nature. Their synthetic biology approach might, among other things, make it possible in the future to optimise biotechnological production processes and to regulate hormonal activity through cell therapy.
Constant despite environmental disturbances
Marine engineers were the first to build such integral feedback control systems, using it to automate ship steering over one hundred years ago. Since then, it has been applied wherever there is a need to keep, say, a direction, temperature, speed or altitude constant and stable in the face of outside influences. The role of integration is that it allows the control system to make corrections based on both the amount and duration of the deviation from the desired constant value.
In biology, too, mechanisms have evolved to maintain, for instance, a constant concentration of substances in the blood. Several years ago, researchers led by Mustafa Khammash, Professor at the Department of Biosystems Science and Engineering, showed that these biological mechanisms are also examples of integral feedback control. “These kinds of integral controllers are extremely resistant to unexpected environmental disturbances,” Khammash says, “which probably explains why the principle prevailed in evolution, and is why it is ubiquitous in technology.”
Interplay of two molecules
Khammash and his interdisciplinary team of control theorists, mathematicians and experimental biologists have now succeeded for the first time in engineering such an integral feedback controller in the form of a synthetic genetic regulatory network inside a bacterium. Their feedback mechanism relies on two molecules – A and B – that bind to each other to become inactive. Together, these two molecules have the ability to maintain a constant concentration of a third molecule, C. The system is designed so that molecule B promotes the production of C, while the production rate of A depends on the concentration of C. The feedback loop consists in the fact that when C is abundant, more A will be produced, which will inactivate more B, which in turn will cause production of C to fall.
As a proof of concept, the ETH scientists made use of this principle to control the production of a green fluorescent protein in Escherichia coli bacteria. Thanks to the feedback controller, the bacteria produced a constant amount of the fluorescent protein – even when the scientists, who wanted to test the system, attempted to suppress its production using strong inhibitors. In a second experiment, the researchers managed to produce a bacterial population that grew at a constant rate in spite of the scientists’ attempts to disrupt growth, again in an effort to test the feedback mechanism.
Improving biotech and therapies
Biotechnology could now put this new control mechanism to work in bacteria to produce vitamins, medications, chemicals or biofuels, with the mechanism ensuring that the production rate within the bacteria is held constant at its optimum level.
The ETH scientists are developing an analogous control mechanism for mammalian cells in subsequent research work, which will pave the way for further applications, including designer cells featuring genetic regulatory networks to produce hormones inside a patient’s body. Among those who would stand to benefit from such an approach are people with diabetes or thyroid deficiency. The synthetic feedback controllers could also be used to improve cancer immunotherapy. “In this form of therapy, immune cells need to be active enough to fight the tumour, but not overactive, as they would then attack healthy tissue,” Khammash says. “A mechanism like ours would be able to fine-tune their activity.”






















































