Almac partners with Merck KGaA and the MRC to assess markers of response to Cetuximab therapy
Almac, Merck KGaA, Darmstadt, Germany, and the Medical Research Council (MRC) have entered into a partnership to undertake a study in metastatic colorectal cancer (mCRC) using samples from the MRC COIN trial. The aim of the study is to assess potential biomarkers of response to cetuximab (Erbitux®).
Almac will utilise samples from the COIN trial to carry out quantitative polymerase chain reaction profiling and data analysis to assess other potential biomarkers in the context of combination therapy with cetuximab.
Prof. Tim Maughan, Director, Wales Cancer Trials Unit, explained: “The MRC COIN trial is the largest trial of therapy in patients with advanced colorectal cancer and tested the addition of cetuximab to oxaliplatin based chemotherapy regimen. This collaboration will enable us to identify whether biomarkers apart from the Kras status alone further define which patients may benefit most from the addition of cetuximab to chemotherapy”
Prof. Paul Harkin, president of Almac Diagnostics, said: “Almac are delighted to once again be involved in such an important study. The use of retrospective analysis from formalin fixed paraffin embedded samples will be one of the keys to successfully delivering better molecular diagnostics, which is paramount in the development of personalised medicine”
The MRC COIN study is a phase III, multicentre, three arm study in 1st-line treatment of mCRC. The study focuses on examining whether the combination of oxaliplatin and cetuximab with fluoropyrimidine chemotherapy improves patient outcomes.
Most read news
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Glucosidases

Hauschild SpeedMixer: Global action against US-based FlackTek Inc. and its European affiliates - Hauschild has filed suit in the US, a civil proceeding in the Netherlands, as well as a criminal proceeding in Spain, against FlackTek and its various agents
'Co-conspirator' cells could hold key to melanoma prediction, prevention
Sever's_disease
T-tubule

Engineering biology is expected to disrupt all industries
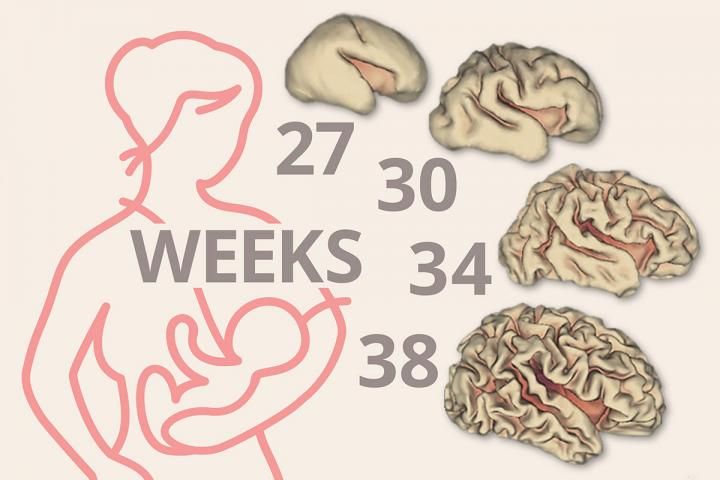
Breast milk linked to significant early brain growth in preemies
Our_Bodies,_Ourselves
Agilent Technologies and French ExonHit Therapeutics to Collaborate - Companies Working to Optimize Microarray Technology for Splice Variant Analysis
InterMed Discovery and Axxam Announce Joint Research Agreement for the Identification of Novel Natural Functional Ingredients
Genome sequence of hypertensive rat expected to uncover the genetic basis of hypertension in humans