Virulence factor of the influenza A virus mapped in real-time
The influenza A viruses, which have instigated deadly pandemics in the past, still remain a major global public health problem until today. Molecules known as virulence factors are produced by bacteria, viruses, and fungi to help them to infect host cells. One of virulence factors found in the influenza A viruses is hemagglutinin (HA). Researchers at Kanazawa University have recently studied the structure of HA of avian influenza virus, H5N1, using high-speed atomic force microscopy (HS-AFM) and the findings are essential for developing therapeutic approaches against influenza A viruses in future.
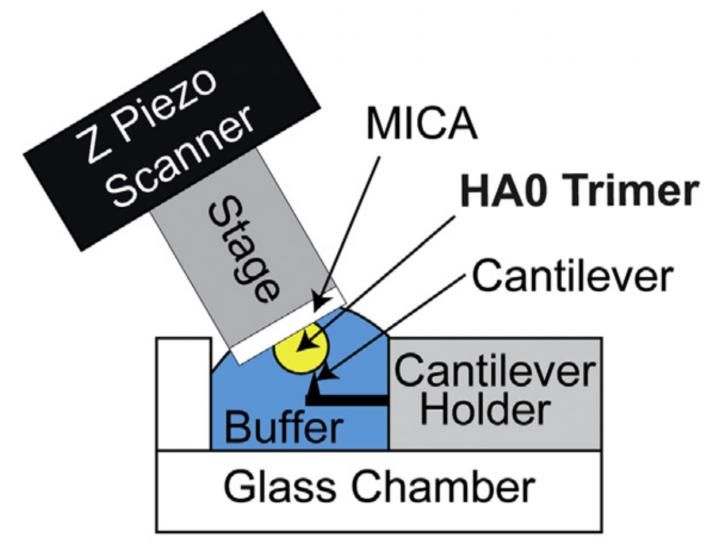
HS-AFM setup for direct visualization of HA0 trimer. Schematic diagram of the HS-AFM setup for scanning the HA0 trimer.
Kanazawa University
HA is initially synthesized by host cells in its precursor form that known as HA0. Conversion of HA0 to HA is depending on the pathogenicity of influenza A viruses: extracellular conversion for low pathogenic influenza A viruses and intracellular conversion for highly pathogenic influenza A viruses. Therefore, understanding the structure and properties of HA0 is paramount to deciphering HA. Richard Wong and his research team thus sought out to scrutinize HA0 under the microscope. Recombinant HA0 protein of H5N1 was visually analyzed by HS-AFM system developed by Kanazawa University.
Both HA0 and HA exist in homotrimeric forms and conversion of HA0 to HA does not significantly modify the homotrimeric structure. Therefore, it is sensible to use HA as a template to generate HA0 HS-AFM simulation images. Acidic endosomal environment is the critical factor for HA to induce fusion between viral membrane and endosomal membrane in order to release viral materials into host cells. To elucidate the acidic effect on HA0, it was first exposed to an acidic environment. The trimer of HA0 turned out to be very sensitive to the acidic solution and expanded considerably. When conformational changes of hemagglutinin were measured in real-time using HS-AFM, the team found that its area was larger, and its height shorter. Acidic environment essentially made the molecule flatter and more circular, as compared to its original counterpart. This change in conformation was, however, reversible as the structure reverted back to its original form upon neutralization.
"Our pilot work establishes HS-AFM as an inimitable tool to directly study viral protein dynamics, which are difficult to capture with low signal-to-noise techniques relying on ensemble averaging, such as cyro-EM and X-ray crystallography," says lead author of the study Dr Kee Siang Lim. "With high scanning speed and a minimally invasive cantilever, we predict that HS-AFM is feasible to reveal the flow of irreversible conformational changes of HA2 induced by low pH, which is mimicking the true biological events that occur when HA enters a host endosome, in future study."
This study paved the way for investigating biological events within viruses in real-time. The authors state the importance of HS-AFM for this research: "Our work establishes HS-AFM as an inimitable tool to directly study viral protein dynamics, which are difficult to capture with low signal-to-noise techniques relying on ensemble averaging, such as cyro-EM and X-ray crystallography," explains Dr Richard Wong, senior author of the study.
Original publication
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Atheroma
Tea_tree_oil
Glomerulus
Category:Genes_on_chromosome_15
Cystic fibrosis: Restoring airway integrity - UNIGE team reveals that hydrating the surface of the airways of people with cystic fibrosis restores their protective barrier against unwanted bacteria

Bactron 400 HP | Anaerobic workbenches | Dunn
Deuteromycota
Category:Genes_on_chromosome_13
Category:Mononegavirales