Micreos Raises €30 Million for Endolysin Technology Set to Replace Antibiotics
Dutch biotechnology company Micreos announced it has secured €30 million in funding to accelerate the development of its endolysin technology, set to replace antibiotics. Proceeds are earmarked for the clinical development program of its endolysin XZ.700 and the USA launch of its OTC Gladskin product for eczema.

Reisefreiheit_eu, pixabay.com, CC0
According to the World Health Organization, antibiotic resistance is one of the world's biggest health threats. The call for true alternatives is more pressing than ever. Antibiotics do not distinguish between bad and good bacteria, and their use induces resistance.
Endolysins have three distinct features: (1) ability to target only unwanted bacteria while preserving the microbiome, which is essential for our health. (2) ability to kill antibiotic resistant strains of bacteria (3) emergence of resistance against endolysins is not expected.
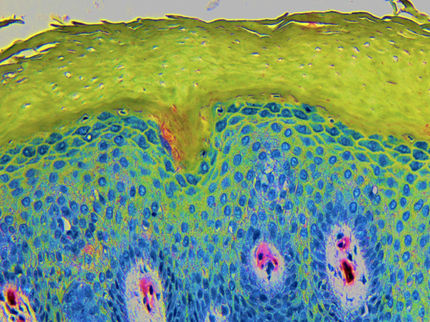
Endolysin technology opens a new therapeutic window for treatment of chronic inflammatory conditions, such as atopic dermatitis and persistent wound infections, including those caused by MRSA.
Micreos' Staphefekt™ SA.100, is the world's first endolysin approved for human use. It selectively targets Staphylococcus aureus which is a major trigger of eczema and other inflammatory skin conditions.
"Since its introduction, Gladskin has helped over 100.000 people in Europe with eczema, acne, and rosacea, many of them reporting a life-changing impact,'' remarked Skyler Stein, Head of Gladskin USA. "Gladskin is pioneering the use of endolysins to rebalance the skin microbiome and improve skin health." Chosen as Europe's Most Impactful Innovation 2018, Gladskin is now preparing its launch in the United States.
The €30 million funding includes a non-dilutive €5.4 million Innovation Credit granted by the Dutch Ministry of Economic Affairs. Micreos has conducted several studies with Gladskin, including trials on eczema, acne and rosacea. Studies in orphan indications with a high unmet need, like primary immune deficiencies and Netherton disease are ongoing.
Micreos CEO Mark Offerhaus noted: "This funding from existing and new investors will advance the adoption of our endolysin technology and help us reach the millions who stand to benefit. We are exploring partnerships to further accelerate the commercialization of our technology."
Most read news
Topics
Organizations
Other news from the department business & finance

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Something is happening in the life science industry ...
This is what true pioneering spirit looks like: Plenty of innovative start-ups are bringing fresh ideas, lifeblood and entrepreneurial spirit to change tomorrow's world for the better. Immerse yourself in the world of these young companies and take the opportunity to get in touch with the founders.