A silver lining like no other
Self-sterilizing microneedles revolutionizing vaccination and drug delivery
Vaccinations are the world's frontline defence against infectious diseases yet despite decades of interventions, unsafe injection practices continue to expose billions of people to serious infection and disease.
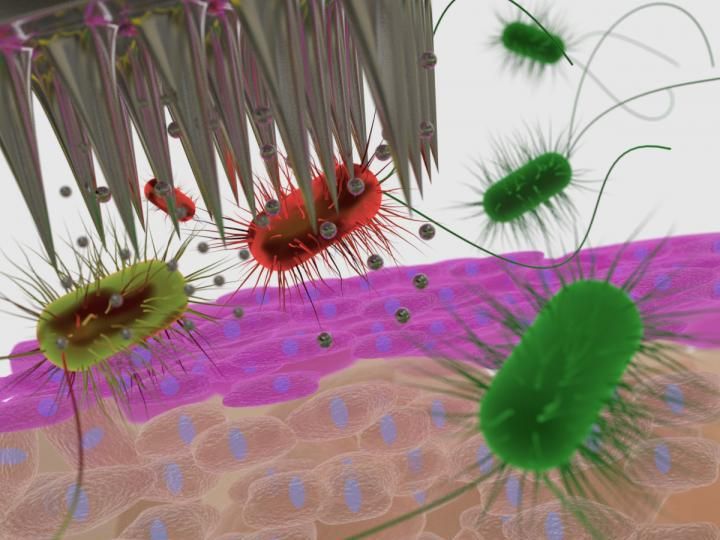
Silver microneedles in action.
University of South Australia
Now, new technology from the University of South Australia is revolutionising safe vaccination practices through antibacterial, silver-loaded dissolvable microneedle patches, which not only sterilise the injection site to inhibit the growth of bacteria, but also physically dissolve after administration.
Lead researcher, Professor Krasimir Vasilev says these first generation microneedles have the potential to transform the safe administration of transdermal vaccinations and drug delivery.
"Injections are one of the most common health care procedures used for vaccinations and curative care around the world," Prof Vasilev says.
"But up to 40 per cent of injections are given with improperly sterilised syringes and needles, placing millions of people at risk of contracting a range of illnesses or diseases.
"Our silver-loaded microneedles have inherently potent antibacterial properties which inhibit the growth of pathogenic bacteria and reduce the chance of infection."
The UniSA study tested the antibacterial efficacy of silver-loaded microneedles against bacteria associated with common skin infections - Golden staph, Staphylococcus epidermis, Escherichia coli and Pseudomonas aeruginosa - and found that the silver-loaded microneedle patches created a 24-hour bacteria-free zone around the patch administration site, a feature unique to the new technology.
The silver-loaded microneedles comprise an array of 15 x 15 needles each 700 micron in length, which pierce only the top layer of the skin without reaching the underlying nerves, making them 100 per cent painless.
The microneedles are made from a safe, biocompatible and highly water-soluble polymer that completely dissolve within one minute of application, leaving behind no sharp waste.
The World Health Organization says that using the same syringe or needle to give injections to more than one person is driving the spread of deadly infectious diseases worldwide, estimating that this may cause up to 1.7 million people to be infected with hepatitis B, 315,000 with hepatitis C, and as many as 33,800 with HIV each year.
Prof Vasilev says the dissolvable feature of the microneedles will significantly improve injection safety.
"Infection from unsafe injection practices occurs all over the world," Prof Vasilev says, "so technologies that protect people from unnecessary infection are critical.
"The dissolvable feature of our silver-loaded microneedles ensures absolutely no risk of reuse, removing one of the greatest causes of infection.
"And by incorporating the antibacterial silver nano-particles into the dissolvable microneedles, we've created a very promising vehicle for safe vaccine and drug delivery around the world."
Original publication
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Category:Microbiology_related_organisations
3D model of human placenta developed

Molecular machine in nano cage - What a toy: a tiny gyroscope that would fit in a human cell and that can be controlled from the outside
Fear_of_youth

Special Software Enhances Accuracy of Genome Annotation for Animals and Plants - "BRAKER3 marks a significant development in bioinformatics and provides academics all over the world access to a high-performance tool for genome annotation"
Blinking neurons give thoughts away
List_of_maize_diseases
In the active centre of carbon dioxide conversion - Scientists reveal the features of efficient carboxylase enzymes
Discovery of a new reprogramming mechanism for tumor cells

From pasture to patient: Scientists distill cow’s milk into nano-capsules for drug delivery - New method to purify exosomes from cow's milk for nano-sized drug delivery capsules - Virginia Tech spin-out company formed