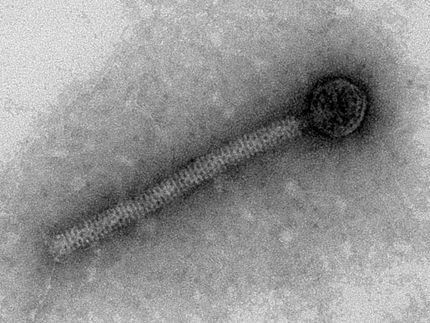
Antibiotic resistances spread faster than so far thought
Resistance genes hop around the genome
By studying fish raised in aquaculture, researchers from the Helmholtz Zentrum München, the University of Copenhagen and the University of Campinas in Brazil have shed new light on the mechanisms by which antibiotic resistance genes are transferred between bacteria. According to the study published in the journal ‘Microbiome’, those mechanisms are more varied than previously thought.

Piaractus mesopotamicus, a South American species known as pacu, is often raised in aquaculture.
© Helmholtz Zentrum München
“In the past 70 years, the use of antibiotics in human and veterinary medicine has steadily increased, leading to a dramatic rise in resistant microorganisms,” says Prof. Dr. Michael Schloter, head of the Research Unit for Comparative Microbiome Analyses (COMI) at Helmholtz Zentrum München. It is especially alarming that many microorganisms are resistant not just to one antibiotic, but to a whole range of different substances, states the corresponding author of the recent study. This poses particular problems in the treatment of infectious diseases. “We therefore set out to discover the mechanisms responsible for resistance development,” he says.
To this end, he and his team, together with Danish scientists led by Gisle Vestergaard (University of Copenhagen and Helmholtz Zentrum München), investigated fish raised in aquaculture. Specifically, they studied Piaractus mesopotamicus, a South American species known as pacu that is often raised in aquaculture. The fish received the antibiotic florfenicol in their food for 34 days. During this time and after the application period, the researchers took samples from the digestive tract of the fish and looked for relevant genetic changes in the gut bacteria.
Resistance genes hop around the genome
“As expected, administration of the antibiotic induced an increase in the genes responsible for resistance to that antibiotic,” explains COMI doctoral student Johan Sebastian Sáenz Medina, lead author of the paper. “One example are genes for pump proteins, which simply remove the active substance from the bacteria again. However, we were particularly surprised by the different mechanisms that we could detect by which antibiotic resistance genes are spread amongst gut bacteria of the fish” Sáenz Medina explains. “This suggests that the bacteria also exchange resistance through viruses, known as phages, and transposons.”
Further metagenomic studies confirmed that these mobile genetic elements induce a fast distribution of resistance genes among genomes of different organisms. So far it has been postulated that only plasmids (in essence, easily transferable mini-chromosomes) are mainly responsible for the exchange of resistance genes.
“The finding that resistance is also extensively transferred between bacteria without the involvement of plasmids is really quite surprising,” says Michael Schloter. “Based on this observation, relevant dissemination models should be reviewed and modified. In addition, our data certainly lead us to question whether and to what extent we should continue to use antibiotics in the world’s increasing number of aquacultures.”
Original publication
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Category:Phototrophic_bacteria
Bacterial cells can tell the time - Chronobiologists report that soil bacteria possess an internal clock

Atomic map of malaria drug gives it new life
Darius_Goes_West

ZEISS to acquire LENSO Sp. z o.o. to strengthen market access and industry expertise in 3D metrology and inspection solutions in Poland
Nanocarrier with Escape Reflex - Tumor-specific drug release through controlled endosomal escape
Mondor's_disease
Newly developed COVID vaccine from Austria could protect against omicron and other variants - Vaccine developed at MedUni Vienna delivers promising data
National_Childbirth_Trust
American_Psychological_Association