From Sea to Lab
Total synthesis of marine antitumor agents trabectedin and lurbinectedin
With its vast numbers of different lifeforms, the sea is a largely unexplored source of natural products that could be starting points for new pharmaceuticals, such as the antitumor drugs trabectedin and lurbinectedin. Because only tiny amounts can be obtained from sea organisms, synthetic production is necessary. In the journal Angewandte Chemie, scientists have introduced a new, efficient synthetic route for these two drugs. A key step is the light-controlled activation of a carbon–hydrogen bond.
© Wiley-VCH
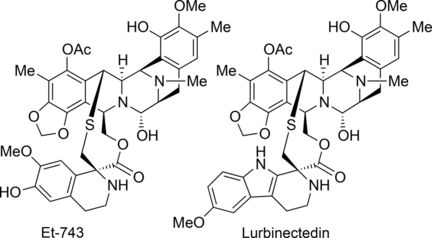
Trabectedin (also called ecteinascedin) comes from the sea squirt species Ecteinascidia turbinata and is the first marine natural product to be used clinically as a drug—for the treatment of advanced soft tissue sarcoma. Lurbinectedin has a slightly modified structure and is currently in phase III clinical studies for the treatment of certain lung and breast cancers. One ton of sea squirts are needed to acquire about one gram of trabectedin. A viable and efficient synthetic route for making this and related drugs in sufficient quantities is therefore needed urgently. However, trabectedin has thus far proven to be one of the most challenging target molecules in natural products synthesis. Various synthetic routes have been proposed but none is really viable. Current methods are very complex, require expensive and uncommon reagents, and deliver unsatisfactory yields.
Researchers working with Dawei Ma at the Shanghai Institute of Organic Chemistry (China) have now described a more efficient and viable de novo synthetic route for trabectedin and lurbinectedin. De novo, also known as total synthesis, means that the natural product is completely synthesized from small, common starting materials.
The synthesis starts with the amino acid S-tyrosine and consists of 26 individual steps. First, several steps are used to produce an intermediate, which acts as the starting material for the separate production of the two halves of the target molecule—Trabectedin or Lurbinectedin. These are then bound together in a later reaction step.
The key step of the synthesis is the light-controlled activation of a normally unreactive carbon–hydrogen bond (remote C–H activation). A radical rearrangement mechanism leads to a ring closure in which a quinone group is converted into a 1,3-benzodioxole unit, which is a structural component found in many natural products. The reaction was particularly efficient under irradiation with blue light in tetrahydrofuran solvent.
The scientists hope that their synthetic route offers a practical and economical method for the production of trabectedin and lurbinectedin, finally providing adequate supplies of these complex marine antitumor drugs.
Original publication
Other news from the department science
These products might interest you
Pharmaceutical Substances by Thieme Verlag
Look up Industrial Syntheses of 2,600 APIs
Your tool for Syntheses, Patents and Applications – Pharmaceutical Substances

KNAUER IJM NanoScaler by KNAUER
Efficient formulation of lipid nanoparticles for RNA-based therapies
Optimise drug encapsulation from 1 ml to hundreds of millilitres with minimal drug input

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
More news from our other portals
Last viewed contents

Spin-off from Charité want to advance AI-powered pathology - Aignostics Raises €5 Million Seed Round
Site-directed_mutagenesis
Chemical_Weapons_Convention
Congenital_disorder
Leukonychia
Category:National_Institute_for_Occupational_Safety_and_Health