Preventing Clumping: How to Keep Proteins Active
Protein molecules are nature’s all-rounders and carry out their specific tasks with great efficiency. For example, some play an important role in converting substances; others are involved in combatting pathogens. As a result, these biological machines are important in technical as well as medical applications. However, proteins tend to clump together irreversibly at high concentrations and thus lose their effectiveness, although this only occurs when they are outside their natural environment of the cell’s interior.
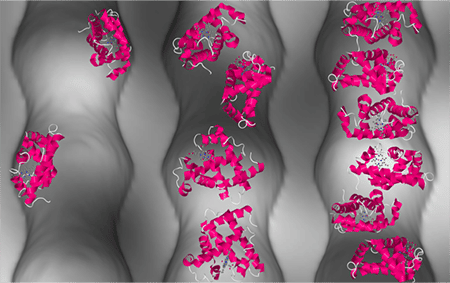
The image illustrates the arrangement of proteins (depicted in pink) in nanopores at various concentrations.
Copyright: Forschungszentrum Jülich
Spatial restrictions can maintain the activities of concentrated protein solutions. Just why this happens has until now been poorly understood. Scientists at Forschungszentrum Jülich and University College London have now demonstrated for the first time that despite high concentration levels, proteins do not clump together in nanocavities of porous silicon dioxide, but instead behave like a fluid. The scientists discovered that crucial to this behaviour are on the one hand the interactions between the proteins and the interface, and on the other, the curvature of the nanopores.
Small-angle neutron scattering at one of the Jülich installations at the Heinz Meier-Leibnitz Zentrum in Garching has enabled the researchers to directly identify the arrangement of the proteins tested, myoglobin and lysozyme, through the nanostructured materials. In doing so, the researchers showed that the method enables connections between the characteristics of the pores, the arrangement of the proteins and their activity to be revealed. The knowledge gained can be used for the development of new and better bioinspired applications in biotechnology, medicine and catalytic processes.
Original publication
Original publication
Justin Siefker, Ralf Biehl, Margarita Kruteva, Artem Feoktystov, Marc-Olivier Coppens; "Confinement Facilitated Protein Stabilization As Investigated by Small-Angle Neutron Scattering"; J. Am. Chem. Soc.; 2018, 140, 40, 12720-12723
Organizations
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.





















































