‘Bio-fishing’ for rare earths
How protein fragments can be used for the recycling of electronics waste
Without important key elements such as copper or rare earth metals, the electronics industry would grind to a halt and electricity would cease to flow. End-of-life products like discarded energy-saving lamps, mobile phones and computers could provide an important secondary source for these valuable elements; however, they are difficult to recover. Unless, that is, small protein fragments are used to ‘fish’ them out – a technique described by researchers at the Helmholtz-Zentrum Dresden-Rossendorf (HZDR) and the TU Bergakademie Freiberg in an article published in the specialist magazine Research in Microbiology.
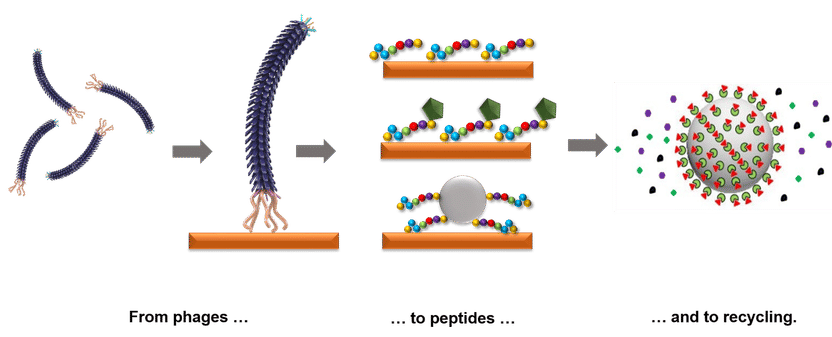
Bacteriophages as a tool for developing selective materials based on peptides. Strategic raw materials can be recovered using peptides (center). In order to produce these protein fragments for each target material (orange), the HZDR junior research group BioKollekt applies bacteriophages - viruses specialized in infecting bacteria (left). Attached to a carrier material (right) the peptides fish the desired metal out of a solution.
Dr. Lederer, Franziska
Nowadays, non-functional energy-saving lamps and old mobile phones can be safely disposed of in special collection bins at supermarkets and other locations, from which they are transferred to smelters where individual components can be salvaged. There are, however, still no economic technologies for recycling the more valuable materials such as the rare earths terbium, yttrium and lanthanum, which are often present in small concentrations and bonded with other components. “By the year 2020, we should be in a position to salvage 25,000 tonnes of fluorescent powder from discarded energy-saving lamps in the European Union alone,” says HZDR biologist Franziska Lederer.
Exotic-sounding compounds such as yttrium oxide, lanthanum phosphate, cerium magnesium aluminium oxide and barium magnesium aluminium oxide, which contain traces of terbium, cerium, europium and other rare earths, are finely dispersed in the powder. The rare earths themselves, which are essential for manufacturing plasma screens, wind turbine generators and electric motors for hybrid cars, have been mined almost exclusively in China in recent years. Export restrictions can therefore adversely affect key technologies on our continent.
The junior research group BioKollekt, which Franziska Lederer has been heading since 1st October 2018 at the Biology Department of Helmholtz Institute Freiberg for Resource Technology (HIF) – a member institute of HZDR – is therefore working on new technologies that can be used to extract rare earths from the fluorescent powder of discarded energy-saving lamps and other similar sources. These methods can also be used to extract important metals such as copper and gold from the tailings of mines or to sort and reuse plastics.
Franziska Lederer models these techniques on viruses that specialize in infecting bacteria. The shell of these tiny ‘bacteriophages’ consists of about 4,000 proteins. Molecular biological methods have been developed to attach short protein fragments to them. These fragments (peptides) are between eight and 16 protein building blocks in length. There are many different sorts of peptide, which means that Franziska Lederer is able to use a billion bacteriophages in her research, each of which has different peptides. Molecular biologists refer to such a collection as a ‘library’.
“The peptides can form small pockets into which certain mini-structures are able to fit,” she explains. A case in point is the rare earth element terbium. Franziska Lederer brings her bacteriophage library into contact with a pure terbium compound which is attached to a solid surface. The surface is then washed, but the bacteriophages and the terbium compound that is a perfect fit for their peptide pockets remain attached.
Reproduction to the point where the perfect fit is found
In a second run-through, the researchers then refine the conditions so that only those bacteriophages with a peptide pocket for which the terbium compound is a perfect fit remain attached. They then analyze the section of the genome of these bacteriophages that contains the blueprint for the peptide. Using this blueprint, Franziska Lederer then concocts the appropriate peptides for the terbium compound.
The peptides are now attached to particles of a magnetic material. If these particles are mixed with the fluorescent powder of energy-saving lamps in a solution, they then attach themselves to the terbium compounds therein. The researchers now use a magnet to ‘fish out’ the particles together with the rare earths. After removal of the terbium compounds, the particles with the peptides can be reused on the next cycle. “With this method, we can obtain specific peptides for various rare earths as well as important metals such as copper, gold and some platinum metals, allowing us to extract the respective substances from highly dilute and complex solutions,” explains Franziska Lederer.
The inventors of the phage display method were awarded the Nobel Prize for Chemistry in 2018. But long before that, Franziska Lederer had received the bacteriophage libraries from the group of the Nobel laureate George Smith at the University of Missouri. As other scientists have hitherto only used the phage method for biological processes such as the production of antibodies, HZDR researchers are veritable trailblazers in the recycling of metals.
Peptides can also be attached to styrofoam beads. The beads float to the surface in a water container with the valuable metals attached and can easily be skimmed off. With such methods, valuable ores can be obtained from mine tailings in which traces are still to be found.
“It may be that we can also isolate peptides that specifically bind certain plastics,” says Franziska Lederer speculatively. Plastic waste frequently ends up being incinerated because it contains a mixture of different plastics. However, the peptides grown by the HZDR research group may one day be able to sort through this mixed waste and thus facilitate real recycling. “Our research is still in its infancy, and a practical application will take some time. We aim to use our innovative technology platform to significantly improve recycling.”