How infectious bacteria hibernate through treatment
Researchers find that bacteria may opt for persistence as well as resistance to antibiotics
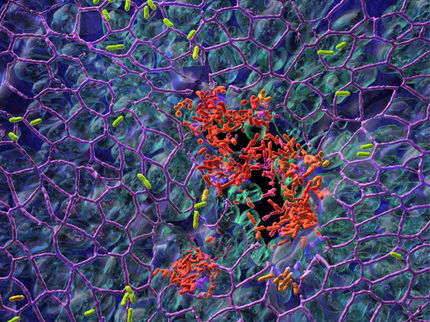
Disease-causing bacteria can develop resistance to antibiotics – which are then no longer effective in treating the infection. Yet they also have another tactic to avoid being killed off by antibiotic treatment. Some cells of the population quietly hide in a dormant state and wait for the danger to subside. Then they return to full function. For example, some urinary tract infections flare up again even after apparently successful treatment with antibiotics. Maja Semanjski, Katrin Bratl and Andreas Kiessling, led by Professor Boris Maček of the University of Tübingen’s Proteom Centrum, and in collaboration with Elsa Germain and Professor Kenn Gerdes of the University of Copenhagen, have investigated such persistent forms of E. coli bacteria. Variations of an enzyme indicated which processes initiate the dormant state. This provides the researchers with possible starting-points at which to seek active substances to combat the dormant cells.
When bacteria become insensitive to a drug, they are said to be resistant. That is the case when, for instance, bacteria can successfully expel antibiotics which have managed to enter their cell; when they become able to break down antibiotics using enzymes; or when they are able to replace one metabolic function blocked by antibiotics with some alternative. “Antibiotics are usually directed at the bacterial growth processes,” Kenn Gerdes explains. He says the bacterium in its dormant phase is not resistant – it is merely temporarily able to tolerate the antibiotic by halting its growth. “Genetically, these bacteria have all the same features of the other bacteria in the population,” and there are no recognizable rules as to which cells in the colony will survive in a dormant state, he adds. “It is rare and affects only one in ten thousand to one million cells. That makes it hard to investigate,” Gerdes says.
Seeking causes for the dormant state
The formation of dormant states is seen as an attribute of persistence. Some clues were offered several years ago by bacteria which were isolated from patients with urinary tract infections caused by Escherichia coli. They had mutations which gave them up to one thousand times greater persistence – without having any greater resistance to antibiotics. A similar discovery was made in cystic fibrosis patients who were infected with the inflammatory Pseudomonas aeruginosa bacterium. One enzyme which can lead to the development of a dormant phase had mutated. It is called the HipA kinase (high persister gene A).
In the current study the research team compared the normal HipA kinase Escherichia coli bacteria with the bacteria which had the mutation. They conducted proteome analyses which recorded all proteins found in the bacterial cells. “We found that the normal and the mutated HipA kinase acted on a different stock of bacterial proteins, modifying them with phosphate,” Boris Maček says. The researchers suspected that the HipA kinase reduces bacterial cells’ growth, initiating the dormant phase. “But the background is more complex than that,” Maček adds. “Our investigations show that the suppression of growth and the persistence are the results of two different processes, because the mutated HipA kinase suppresses bacterial cell growth far less strongly than the normal one does, yet it raises the persistence many times over.” This suggests new processes in the cell which future medicines will have to tackle to beat dangerous degrees of persistence in infectious bacteria.
Original publication
Most read news
Original publication
Maja Semanjski, Elsa Germain, Katrin Bratl, Andreas Kiessling, Kenn Gerdes and Boris Macek; "The kinases HipA and HipA7 phosphorylate different substrate pools in Escherichia coli to promote multidrug tolerance"; Science Signaling; 11, eaat5750 (2018).
Topics
Organizations

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Marius_Romme