Questioning conventional understanding of antifreeze proteins
Scientists describe new phenomenon possibly expanding application
Advertisement
Scientists have discovered that an ice-binding protein (fcIBP) from the sea ice microalga does not fit in the conventional classification of ice-binding proteins, suggesting unknown mechanisms behind its antifreeze property. This finding could lead to a broader application of the antifreeze protein in food and medical industries.
Maddalena Bayer-Giraldi
Organisms living in cold zones produce ice-binding (antifreeze) proteins to prevent themselves from freezing to death. Such proteins have been classified in two groups; the hyperactive type attaches to the hexagonal basal faces of ice crystals to inhibit ice crystal growth and lowers the freezing temperature by up to six degrees C while the moderate type does not attach to the basal faces and lowers the freezing temperature by not more than 1 degree C.
“Many studies on ice-binding proteins have centered on biochemical perspectives, but these proteins have only recently been researched from the viewpoint of crystal growth physics,” says Professor Gen Sazaki of the research team at Hokkaido University.
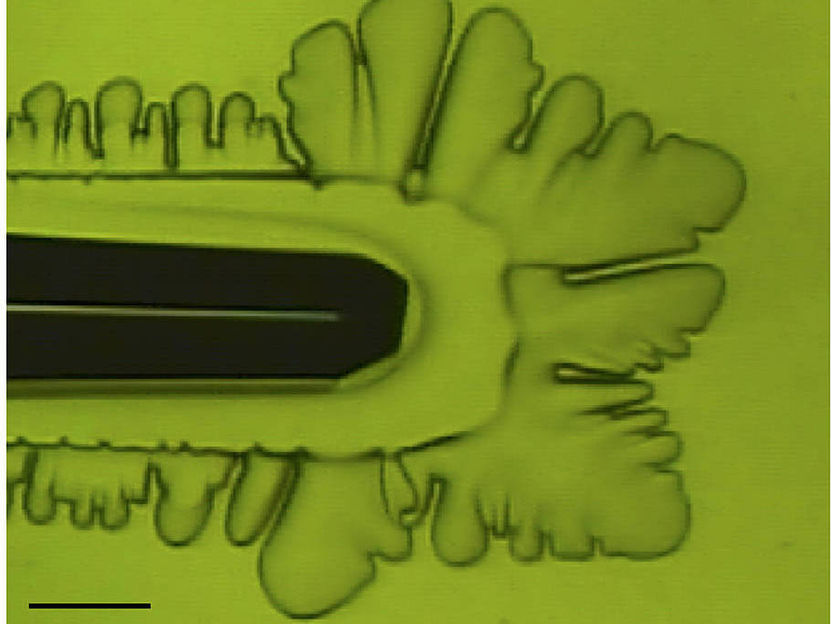
The researchers used their original chamber developed at Hokkaido University’s Institute of Low Temperature Science, that allowed them to observe in detail the growth of ice crystals in water. The morphology of ice crystals to which fclBP had attached was observed under microscopes and their growth rates were precisely measured.
“To our surprise, we found that fclBP – which is known to be effective in lowering the freezing point by less than 1 degree C – attaches to both basal and prism faces, thus affecting ice crystal growth”, says Dr. Maddalena Bayer-Giraldi, first author from the Alfred-Wegener-Institute, Helmholtz Centre for Polar and Marine Research (AWI). When the water temperature was not very low, crystal growth was inhibited and ice crystals became faceted, appearing as hexagonal plates, a phenomenon never seen in ice crystals in pure water. When the water temperature was sufficiently low, the ice crystals took a normal dendrite form. But because fclBP suppressed ice crystal growth on the prism faces, the dendrite branches became narrower, allowing the easier release of heat and thus the faster growth of the tips of the crystal branches.
The study showed that fclBP attaches to both basal and prism faces of ice crystals although it is capable of lowering the freezing point by less than 1 degree C or so, defying the conventional classification of ice-binding proteins. “Ice-binding protein functions cannot be evaluated only by the attachment of the proteins to basal faces or by ice crystal growth inhibition. We need to understand the molecular mechanisms behind their antifreeze properties. Greater understanding of ice-biding proteins could lead to their application in the preservation of food and living organs as well as in cryosurgery,” says Dr. Maddalena Bayer-Giraldi.
The team also included Dr. Dmitry A. Vorontsov of Lobachevsky State University of Nizhny Novgorod in Russia and conducted the research at the Institute of Low Temperature Science at Hokkaido University.






















































