Growing brain cancer in a dish
Organoids, that mimic the onset of brain cancer
brain tumors are among the most aggressive and deadly cancers and a leading cause of cancer-related death in children and young adults.
Glioblastoma is a particularly aggressive form of brain tumor that is characterized by a very rapid tumor growth of glia cells – the most abundant cell types in the central nervous system, that surround and support neurons. The tumor is notoriously difficult to treat as it forms tentacle-like structures, that makes it difficult to remove the tumors surgically. Also, glioblastoma tend to liaise with blood vessels, helping cancerous cells to grow and spread very quickly. With each cell division, they accumulate mutations that can render them resistant to drugs. Because of the molecular characteristics of the human brain, it has been very difficult for scientist to find a suitable model organism to study brain cancers.
Organoid technology could now lead to a paradigm shift in studying glioblastoma and other forms of brain cancers. In recent years, researchers have managed to develop more and more lab grown organ like structures, or organoids. Already in 2013 Jürgen Knoblich and his team at IMBA were the first ones to use brain- organoids from iPS cells for studying molecular and developmental characteristics as well as neurological diseases of the human brain. Now, they have succeeded to grow one of the deadliest cancers, brain cancer, in a dish. The novelty: this method allows the researchers to replicate the process of brain carcinogenesis in a dish, where they can observe brain cancer onset already at its early stages and watch the brain-organoid growing a tumor in a dish.
The group reports on the new neoplastic cerebral organoids they have developed to study brain tumors. These organoids faithfully reproduce unique aspects of the human brain, such as its diverse cell types and the stages by which it develops, allowing scientists to get a handle on the ways tumors arise, and also provide a system to try out new cancer therapies. “For the first time, we established a human model for brain cancer to rapidly test or screen for the tumorigenic potential of gene mutations found from cancer genome sequencing projects. Using genome-editing techniques, we introduced clinically-relevant mutations into the cerebral organoids. With this method, we are trying to mimic the brain tumor onset in vivo by only mutating a very small population of cells,” explains Shan Bian, postdoc at IMBA and first author of the study.
Over the past few years, huge cancer genome sequencing projects have catalogued thousands of mutations found in patient tumors. Such genetic defects arise through natural mistakes in the copying of DNA, through the consumption of carcinogens or other causes. They trigger fundamental changes in healthy cells that cause them to spin out of control, reproducing at an astounding pace. Each time such a cell divides it may generate new mutations, leaving scientists with a huge puzzle to assemble. The novel brain-cancer or neoplastic organoids now offer a means to investigate, how some of these mutations are driving forces in tumors, crucial in triggering the onset of cancer, and necessary to address through treatments, while others are simply side effects.
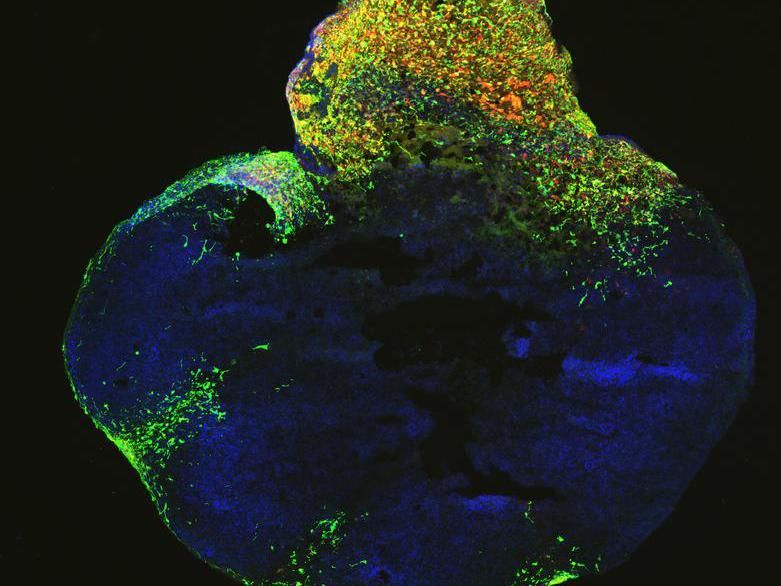
Neoplastic cerebral organoid with GFP-positive tumor regions (green), which demonstrates glioblastoma-like cellularity.
IMBA
From bench to bedside
The scientists carried out studies of these miniature tumors to identify additional changes they had undergone, identifying new markers that may help in diagnosing the disease and classifying the tumor a patient has.
Once a tumor has developed, the scientists can target a specific mutation to determine whether the defect is also essential for the long-term survival of the tumor. Any change that causes it to shrink or disappear makes a good candidate for future therapies.
The scientists tested this principle by applying a drug called Afatinib, which is currently being used in clinical trials as a treatment for glioblastoma. They found that after 40 days, administration of the drug significantly reduced the number of tumor cells in two combinations of mutations, but not in the third or in cells with too much MYC. Afatinib inhibits a molecule called EGFR – which is specifically overexpressed in those two types of tumors. When testing four additional EGFR inhibitors used in therapies, a drug called Erlotinib significantly reduced the number of tumor cells as well, while the effects of others were minimal.
"These results show that brain organoids also have significant impact for cancer research and public health. We can now develop organoids from brain tumor patients allowing us to test the efficacy of different combinations of therapies," says stem cell pioneer Knoblich, who is interim director of IMBA. "As a next step it will be important to foster clinical partnerships, so we can work towards translating our findings from the bench to the bedside."


















































