Enigma of fatty acid metabolism solved
Enzyme shape controls its activity
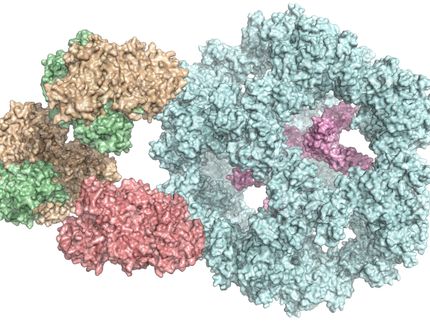
fats are essential for our body. The core components of all fats are fatty acids. Their production is initiated by the enzyme ACC. Researchers at the University of Basel’s Biozentrum have now demonstrated how ACC assembles into distinct filaments. As the researchers report in “Nature,” the type of filament formed controls the activity of the enzyme and thus fatty acid production.
ACC filaments regulate enzyme activity and thus control fatty acid production.
University of Basel, Biozentrum
Fats are highly diverse molecules that serve as fuel and energy storage, and they constitute the building blocks for cell membranes, hormones and messengers. Despite the diversity of fats, all the fatty acids contained therein arise from the same precursor. Only a single enzyme initiates its production: acetyl-CoA-carboxylase, ACC for short. ACC is therefore the linchpin of fatty acid synthesis and understanding the architecture of ACC is critical for treating many diseases.
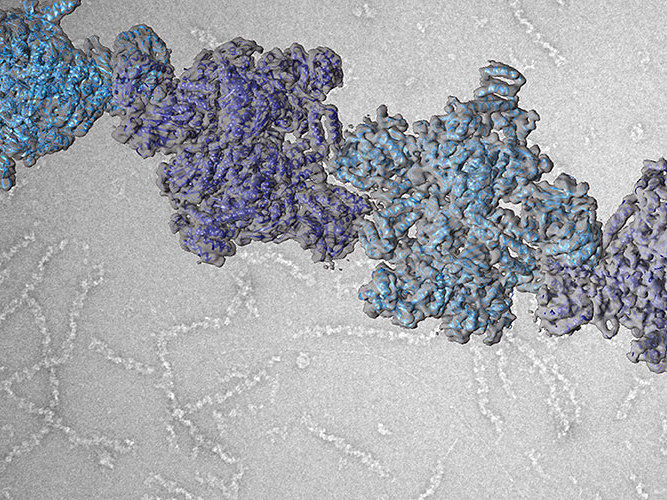
While the enzyme and its function in metabolism have been known for nearly sixty years, scientists have understood very little about the structure of ACC. In fact, modern biochemistry textbooks continue to show old and blurry pictures of filaments formed by ACC, leaving the how and why of filament formation an enigma. Now, a team of researchers led by Prof. Timm Maier from the Biozentrum of the University of Basel has sharpened the picture. “We have solved this long-standing puzzle in metabolism,” reports Maier. “Elucidating the detailed architectures of ACC filaments revealed their impact on enzymatic activity.”
Pacemaker of fatty acid synthesis
ACC is a key regulator of metabolism and the pacemaker enzyme of fatty acid production. Hence, the regulation of ACC activity is highly complex. Only about half of the ACC enzyme catalyzes chemical reactions while the other half is responsible for controlling ACC activity, acting as a sensor for the demand for ACC products and serving as on-off switch of the enzyme.
Shape of ACC determines its activity
ACC activity is not always the same. Depending on its shape, the activity is high or low. Metabolites signaling an excess of carbohydrates drive the enzyme into its active state. “Dozens of ACC enzymes are linked to form a single filament,” says Maier. “In this filament, the enzymatic domains are stably arranged to functionally interact with each other. Only then can ACC efficiently catalyze chemical reactions and stimulate fatty acid production. When ACC is not integrated into a filament, the enzymatic domains are flexibly linked and do not collaborate productively.” ACC can also be switched off by filament formation. Specific control factors force ACC to form inactive filaments, in which the enzymatic domains are strictly separated. This versatile mode of regulation by changing the overall shape of the enzyme is unique and was previously unknown.
ACC as a target structure for drug development
Due to its crucial role in metabolism, ACC is an important target for drug development. Inhibiting ACC activity has the potential to combat cancers or certain viral infections because rapidly proliferating tumor cells and membrane-enveloped viruses require a particularly large amount of fatty acids as membrane components. ACC may also serve as a target for controlling risk factors for developing cardiovascular disease and diabetes linked to aberrant lipid and carbohydrate metabolism, summarized as “metabolic syndrome.” This study opens new possibilities for the development of selective ACC inhibitors that interfere with the activation and filament formation of ACC and ultimately limit fatty acid biosynthesis
Original publication
Moritz Hunkeler, Anna Hagmann, Edward Stuttfeld, Mohamed Chami, Yakir Guri, Henning Stahlberg, Timm Maier; "Structural basis for regulation of human acetyl-CoA carboxylase"; Nature; 2018
Most read news
Original publication
Moritz Hunkeler, Anna Hagmann, Edward Stuttfeld, Mohamed Chami, Yakir Guri, Henning Stahlberg, Timm Maier; "Structural basis for regulation of human acetyl-CoA carboxylase"; Nature; 2018
Organizations
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.