Link between bacteria metabolism and communication could pave way for new antivirulence drugs
Advertisement
Researchers have discovered a link between bacteria metabolism and cell-to-cell communication, potentially providing a target for new antivirulence and antibiofilm drugs.
Effective communication between bacteria could depend on the availability of glucose.
Purdue University
Current drugs for bacterial infections aim to kill bacteria in large quantities, but such drugs are often eventually met with antibiotic resistance. Antivirulence and antibiofilm drugs, on the other hand, block toxic molecules produced by bacteria or prevent bacteria from forming thin, slimy films called biofilms (a common example is dental plaque). These mechanisms are controlled by quorum sensing: the process that allows bacteria populations to communicate and coordinate group behavior.
For example, biofilm forming E. coli can cause urinary tract infections. Thus, disrupting quorum sensing and biofilm forming in the bacteria could prevent those infections.
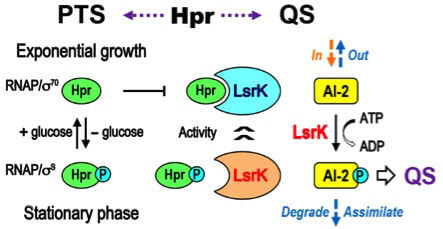
Quorum sensing is facilitated by signaling molecules called autoinducers. One particular autoinducer, AI-2, is modified by a protein called LsrK so that its signal can be perceived.
New research shows that LsrK forms a complex with HPr, a protein involved in glucose utilization in E. coli.
“The fact that they are physically connected provides tantalizing evidence that these two very important pathways actually communicate,” said Herman Sintim, a professor of chemistry and researcher in Purdue University’s Institute for Drug Discovery.
The research team, which included scientists from Korea Basic Science Institute and the University of Maryland, made another surprising discovery: whether LsrK binds to HPr depends on whether there is a phosphate group attached to HPr, which is determined by glucose availability. When glucose is present, the phosphate group on HPr is transferred to the imported sugar, but when glucose levels are low, the phosphate group on HPr is not transferred. Thus, the form of HPr with a phosphate group accumulates.
“We’ve linked the absence of glucose or related sugars to the shutting down of the quorum sensing process,” said Sintim. “When glucose is low, HPr, which now has a phosphate group, binds LsrK less. The uninhibited LsrK can modify AI-2, which facilitates quorum sensing. This means cooperation between E. coli and related bacteria could depend on the availability of glucose.”
If researchers can find other synthetic molecules that bind LsrK and force it into an inactive state, like HPr does, they could prevent some bacteria from sensing their environment and communicating.
“If bacteria go into an environment and other bacteria are producing the language, they are forced to listen,” Sintim said. “But now it looks like we might be able to close their ears.”
Original publication
Ha, Jung-Hye and Hauk, Pricila and Cho, Kun and Eo, Yumi and Ma, Xiaochu and Stephens, Kristina and Cha, Soyoung and Jeong, Migyeong and Suh, Jeong-Yong and Sintim, Herman O. and Bentley, William E. and Ryu, Kyoung-Seok; "Evidence of link between quorum sensing and sugar metabolism in Escherichia coli revealed via cocrystal structures of LsrK and HPr"; Science Advances; 2018