In the beginning was the phase separation
Was a simple mechanism decisive for the origin of life?
The question of the origin of life remains one of the oldest unanswered scientific questions. A team at the Technical University of Munich (TUM) has now shown for the first time that phase separation is an extremely efficient way of controlling the selection of chemical building blocks and providing advantages to certain molecules.
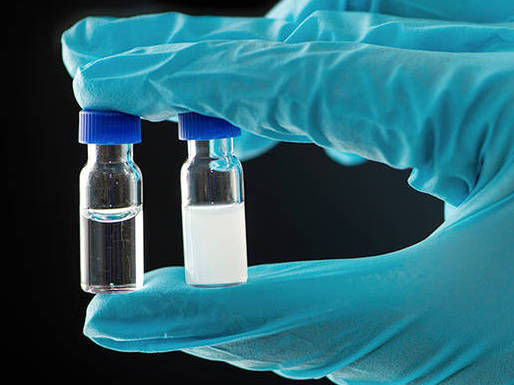
Left: clear solution, right: aqueous solution, clouded by ultrafine oil droplets.
A. Battenberg / TUM
Life needs energy. Without energy, cells cannot move or divide, not even basic functions such as the production of simple proteins could be maintained. If energy is lacking, more complex connections disintegrate quickly, early life would die immediately.
Chemist Job Boekhoven and his team at TUM have now succeeded in using phase separation to find a mechanism in simple molecules that enables extremely unstable molecules such as those found in the primordial soup to have a higher degree of stability. They could survive longer, even if they had to survive a period without external energy supply.
The principle of simplicity
Job Boekhoven and his team were looking for a simple mechanism with primitive molecules that could produce life-like properties. "Most likely, molecules were simple in the primordial soup," says Boekhoven. The researchers investigated what happens when they "fed" various carboxylic acid molecules with high-energy carbodiimide condensing agents, thus bringing them out of equilibrium.
The reaction produces unstable anhydrides. In principle, these non-equilibrium products quickly disintegrate into carboxylic acids again. The scientists showed that the anhydrides that survived the longest were those that could form a kind of oil droplet in the aqueous environment.
Molecules in the garage
The effect can also be seen externally: the initially clear solution becomes milky. The lack of water in the oil droplets is like a protection because anhydrides need water to disintegrate back into carboxylic acids.
Boekhoven explains the principle of phase separation with an analogy: "Imagine an old and rusty car: Leave it outside in the rain, and it continues to rust and decomposes because rusting is accelerated by water. Put it in the garage, and it stops rusting because you separate it from the rain."
In a way, a similar process occurs in the primordial soup experiment: Inside the oil droplet (garage) with the long-chain anhydride molecules there is no water, so its molecules survive longer. If the molecules compete with each other for energy, again those that can protect themselves by forming oil droplets are likelier to survive, while their competitors get hydrolyzed.
Original publication
Marta Tena-Solsona, Caren Wanzke, Benedikt Riess, Andreas R. Bausch, Job Boekhoven; "Self-selection of dissipative assemblies driven by primitive chemical reaction networks"; Nature Comm.; 2018
Most read news
Original publication
Marta Tena-Solsona, Caren Wanzke, Benedikt Riess, Andreas R. Bausch, Job Boekhoven; "Self-selection of dissipative assemblies driven by primitive chemical reaction networks"; Nature Comm.; 2018
Topics
Organizations
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Uganda_Virus_Research_Institute
Category:AstraZeneca
Levomepromazine
Ministry_of_Health,_Labour_and_Welfare_(Japan)
Brief_therapy
Harvey_Bialy
Escherichia_coli_O121
Uranyl_sulfate
Rotary_Scholarships
Transcription_(genetics)