Genes that helps prevent brain disease
Scientists know that faulty proteins can cause harmful deposits or "aggregates" in neurological disorders such as Alzheimer's and Parkinson's disease. Although the causes of these protein deposits remain a mystery, it is known that abnormal aggregates can result when cells fail to transmit proper genetic information to proteins. University of California San Diego Professor Susan Ackerman and her colleagues first highlighted this cause of brain disease more than 10 years ago. Now, probing deeper into this research, she and colleagues have identified a gene, Ankrd16, that prevents the protein aggregates they originally observed.
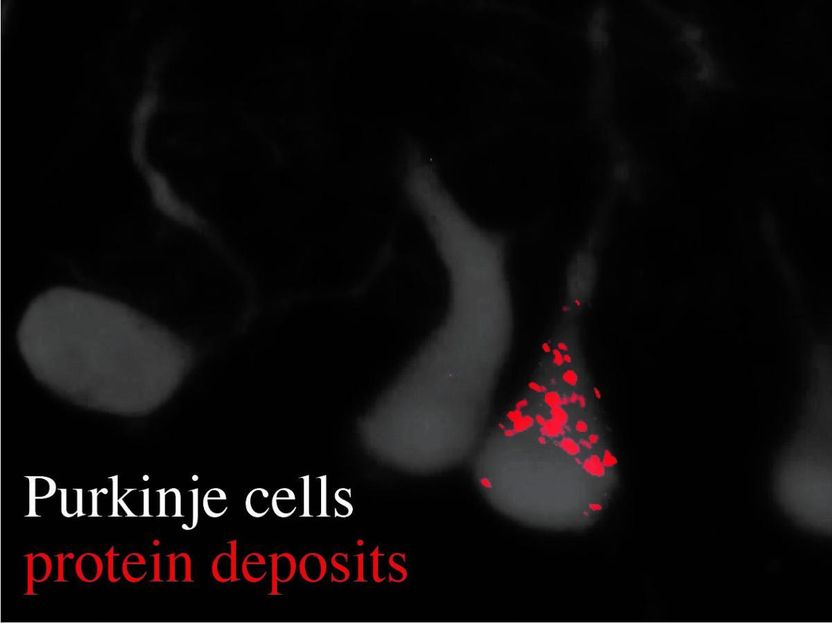
Image reveals Purkinje cells (gray) and their dendrites, as well as an accumulation of protein deposits (red dots).
Ackerman Lab/UC San Diego
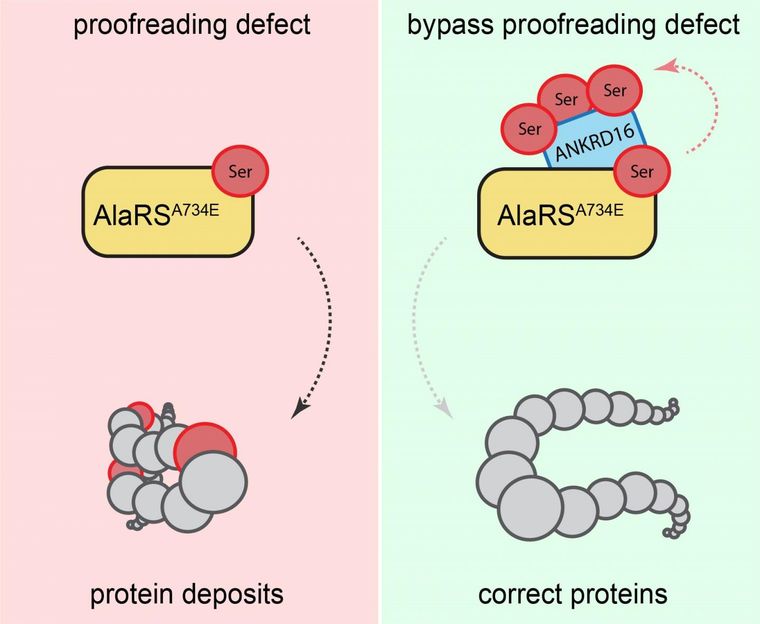
Proofreading defect bypassed.
Ackerman Lab/UC San Diego
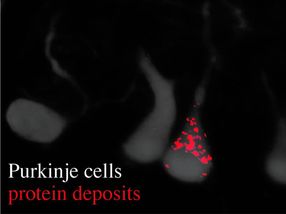
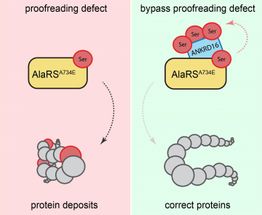
Usually, the information transfer from gene to protein is carefully controlled--biologically "proofread" and corrected--to avoid the production of improper proteins. As part of their recent investigations Ackerman, Paul Schimmel (Scripps Research Institute) My-Nuong Vo (Scripps Research Institute) and Markus Terrey (UC San Diego) identified that Ankrd16 rescued specific neurons--called Purkinje cells --that die when proofreading fails. Without normal levels of Ankrd16, these nerve cells, located in the cerebellum, incorrectly activate the amino acid serine, which is then improperly incorporated into proteins and causes protein aggregation.
"Simplified, you may think of Ankrd16 as acting like a sponge or a 'failsafe' that captures incorrectly activated serine and prevents this amino acid from being improperly incorporated into proteins, which is particularly helpful when the ability of nerve cells to proofread and correct mistakes declines," said Ackerman, the Stephen W. Kuffler Chair in Biology, who also holds positions in the UC San Diego School of Medicine and the Howard Hughes Medical Institute.
The levels of Ankrd16 are normally low in Purkinje cells, making these neurons vulnerable to proofreading defects. Elevating the level of Ankrd16 protects these cells from dying, while removing Ankrd16 from other neurons in mice with a proofreading deficiency caused widespread buildup of abnormal proteins and ultimately neuronal death.
The researchers describe Ankrd16 as "...a new layer of the machinery essential for preventing severe pathologies that arise from defects in proofreading."
The researchers note that only a few modifier genes of disease mutations such as Ankrd16 have been identified and a modifier-based mechanism for understanding the underlying pathology of neurodegenerative diseases may be a promising route to understand disease development.
Original publication
My-Nuong Vo, Markus Terrey, Jeong Woong Lee, Bappaditya Roy, James J. Moresco, Litao Sun, Hongjun Fu, Qi Liu, Thomas G. Weber, John R. Yates III, Kurt Fredrick, Paul Schimmel & Susan L. Ackerman; "ANKRD16 prevents neuron loss caused by an editing-defective tRNA synthetase"; Nature; 2018
Most read news
Original publication
My-Nuong Vo, Markus Terrey, Jeong Woong Lee, Bappaditya Roy, James J. Moresco, Litao Sun, Hongjun Fu, Qi Liu, Thomas G. Weber, John R. Yates III, Kurt Fredrick, Paul Schimmel & Susan L. Ackerman; "ANKRD16 prevents neuron loss caused by an editing-defective tRNA synthetase"; Nature; 2018
Topics
Organizations
Other news from the department science
These products might interest you

Octet R2 / Octet R4 / Octet R8 by Sartorius
Full power on 2, 4 or 8 channels: Label-free and GxP-compliant analysis of molecular interactions
Innovative label-free real-time protein quantification, binding kinetics and rapid screenings

Octet RH16 and RH96 by Sartorius
Efficient protein analysis for process optimisation and manufacturing control in high-throughput
Label-free protein quantification and characterization of protein-protein interactions

Octet SF3 by Sartorius
Surface Plasmon Resonance (SPR) using Single Dynamic Injections for Kinetics and Affinities
Curvature is Key - Adding a ‘Third Dimension’ to the Binding Curve

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.




















































