A new way to stimulate cellular recycling process
Advertisement
Brown University researchers studying the biology of aging have demonstrated a new strategy for stimulating autophagy, the process by which cells rebuild themselves by recycling their own worn-out parts.
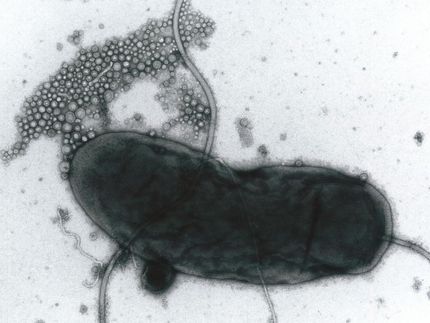
Researchers have found a new way to stimulate autophagy, the process by which cells recycle spare parts. The image shows lysosomes, organelles that break down molecules for recycling, in a human cell line.
Lapierre lab / Brown University
In a study the researchers show that the approach increased the lifespans of worms and flies, and experiments in human cells hint that the strategy could be useful in future treatments for Alzheimer's disease, ALS and other age-related neurodegenerative conditions.
"Autophagy dysfunction is present across a range of age-related diseases including neurodegeneration," said Louis Lapierre, an assistant professor of molecular biology, cell biology and biochemistry at Brown who led the work. "We and others think that by learning how to influence this process pharmacologically, we might be able to affect the progression of these diseases. What we've shown here is a new and conserved entry point for stimulating autophagy."
Autophagy has become a hot topic in recent years, earning its discoverer the Nobel Prize in Physiology and Medicine in 2016. The process involves the rounding up of misfolded proteins and obsolete organelles within a cell into vesicles called autophagosomes. The autophagosomes then fuse with a lysosome, an enzyme-containing organelle that breaks down those cellular macromolecules and converts it into components the cell can re-use.
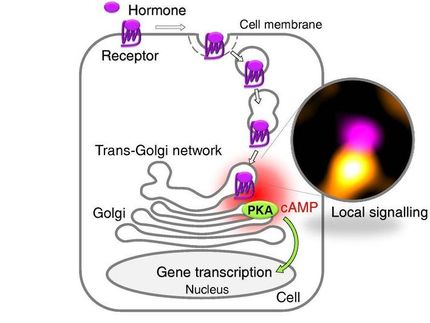
Lapierre and his colleagues wanted to see if they could increase autophagy by manipulating a transcription factor (a protein that turns gene expression on and off) that regulates autophagic activity. In order for the transcription factor to switch autophagic activity on, it needs to be localized in the nucleus of a cell. So Lapierre and his team screened for genes that enhance the level of the autophagy transcription factor, known as TFEB, within nuclei.
Using the nematode C. elegans, the screen found that reducing the expression of a protein called XPO1, which transports proteins out of the nucleus, leads to nuclear accumulation of the nematode version of TFEB. That accumulation was associated with an increase in markers of autophagy, including increased autophagosome, autolysosomes as well as increased lysosome biogenesis. There was also a marked increase in lifespan among the treated nematodes of between about 15 and 45 percent.
"What we showed was that by blocking the escape of this transcription factor from the nucleus, we could not only influence autophagy but we could get an increase in lifespan as well," Lapierre said.
The next step was to see if there were drugs that could mimic the effect of the gene inhibition used in the screening experiment. The researchers found that selective inhibitors of nuclear export (SINE), originally developed to inhibit XPO1 to treat cancers, had a similar effect -- increasing markers of autophagy and significantly increasing lifespan in nematodes.
The researchers then tested SINE on a genetically modified fruit fly that serves as a model organism for the neurodegenerative disease ALS. Those experiments showed a small but significant increase in the lifespans of the treated flies. "Our data suggests that these compounds can alleviate some of the neurodegeneration in these flies," Lapierre said.
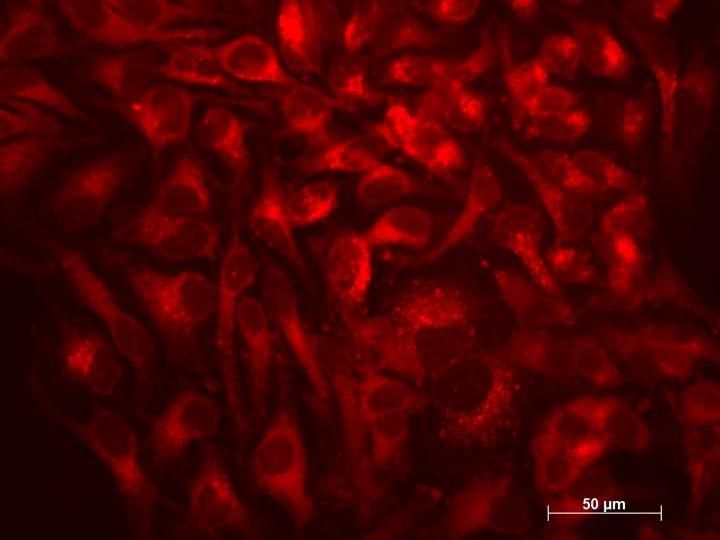
As a final step, the researchers set out to see if XPO1 inhibition had similar effects on autophagy in human cells as it had in the nematodes. After treating a culture of human HeLa cells with SINE, the researchers found that, indeed, TFEB concentrations in nuclei increased, as did markers of autophagic activity and lysosomal biogenesis.
"Our study tells us that the regulation of the intracellular partitioning of TFEB is conserved from nematodes to humans and that SINE could stimulate autophagy in humans," Lapierre said. "SINE have been recently shown in clinical trials for cancer to be tolerated, so the potential for using SINE to treat other age-related diseases is there."
Future research, Lapierre said, will focus on testing these drugs in more clinically relevant models of neurodegenerative diseases. But this initial research is a proof of concept for this strategy as a means to increase autophagy and potentially treat age-related diseases.
Lapierre is a faculty member in the newly approved Center on the Biology of Aging within the Brown Institute for Translational Science. This center, led by Professor of Biology John Sedivy, studies the biological mechanisms of aging. The center's mission is to expand biomedical research and education programs in the emerging discipline of biogerontology, and to bring forth scientific discoveries related to aging and associated disorders.
Original publication
Melissa J. Silvestrini, Joseph R. Johnson, Anita V. Kumar, Tara G. Thakurta, Karine Blais, Zachary A. Neill, Sarah W. Marion, Victoria St. Amand, Robert A. Reenan, Louis R. Lapierre; "Nuclear Export Inhibition Enhances HLH-30/TFEB Activity, Autophagy, and Lifespan"; Cell Reports; 2018