Trial shows chemotherapy is helping kids live with pulmonary vein stenosis
Pulmonary vein stenosis (PVS) is a rare disease in which abnormal cells build up inside the veins responsible for carrying oxygen-rich blood from the lungs to the heart. It restricts blood flow through these vessels, eventually sealing them off entirely if left untreated. Typically affecting young children, the most severe form of PVS progresses very quickly and can cause death within a matter of months after diagnosis.
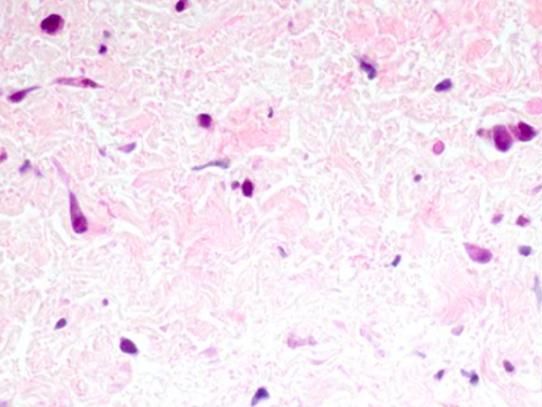
This is a magnification of pulmonary vein tissue showing signs of pulmonary vein stenosis (plump abnormal cells stained dark magenta).
Callahan et al./Boston Children's Hospital
Until recently, treatment options have been limited to keeping the pulmonary veins open through catheterization or surgery. Yet this approach only removes the cells but does nothing to prevent their regrowth. Now, a clinical trial shows that adding chemotherapy to a treatment regimen including catheterization and surgery can deter abnormal cellular growth and finally give children with PVS a chance to grow up.
"Through this approach, we've created the first-ever population of survivors who are living with severe PVS," says Christina Ireland, RN, MS, FNP, who has managed enrolling patients in the trial and treating new patients since the trial ended. "We've changed this disease from an acute killer to a chronic, manageable condition."
The earlier the patients were able to start chemotherapy, the better they are faring now, according to Boston Children's cardiologist and researcher Kathy Jenkins, MD, MPH, who was senior author on the paper. Working with neuro-oncologist Mark Kieran, MD, PhD, of the Dana-Farber/Boston Children's Cancer and Blood Disorders Center, Jenkins brought the innovative chemotherapy approach to PVS patients at the Boston Children's Heart Center.
"In cancer treatment, chemotherapy works by targeting certain receptors on abnormal cells," Jenkins says. "We started down this path because we had reason to believe that PVS cells had similar types of receptors."
Finding common ground in vascular disease
Jenkins, Kieran and a team of collaborators zeroed in on receptors for platelet-derived growth factor and vascular endothelial growth factor as targets that two types of leading chemotherapies (imatinib mesylate and bevacizumab) are able to attack.
"By targeting these growth factor receptors with chemotherapy, we hypothesized that we could be successful in slowing down the abnormal cellular buildup caused by pulmonary vein stenosis," says Ryan Callahan, MD, a pediatric cardiologist at Boston Children's and the first author on the paper.
Given the promise of the approach, the Food and Drug Administration initially approved the clinical trial to include ten patients as a pilot. Soon, however, patient responses showed that the approach was working without undue toxicity. Consequently, the trial expanded to treat 48 total patients over the course of five years.
"By the 48th week of treatment, we were able to stabilize PVS progression and prevent further lung damage in 31 percent of the children we treated," Jenkins says. "At 72 weeks, 77 percent of patients treated per our protocol were still alive. This is truly remarkable when you consider that historically, children diagnosed with severe PVS have been given mere months to live."
Racing to provide treatment in time
Jenkins says that when a patient presents with acute symptoms of PVS, the heart and lungs are both at jeopardy.
"The lungs need ventilation and blood flow to survive," Jenkins says. "When patients reach a point where their PVS has progressed to a severe enough degree, it's possible for them to lose function of one or both lungs. Additionally, keeping the veins clear is critical to ensure that the heart isn't under dangerous strain from struggling to pump blood."
Ireland agrees. "From the children we've treated in our trial, we've quickly learned that early intervention is the key to preventing permanent cardiac scarring and lung damage that can impact their long-term quality of life," she says.
Ireland has played a special role in the success of the trial. Jenkins and other colleagues say she stopped at nothing to bring nearly 50 young patients and their parents from all over the U.S. to Boston to receive treatment, even sending out a medical jet to get them when necessary.
Going above and beyond
After the patients arrived and were medically cleared to receive treatment, Ireland helped navigate the road ahead. She worked to get the experimental therapy approved by families' insurance companies and coordinated every aspect of each child's care. For children who were too young to swallow the chemotherapy pills, Ireland showed parents how to crush the pills and add them to apple juice.
"A clinical trial served in a juice glass!" Ireland has said with a laugh.
She gave out her personal cell phone number to every single child's parents so that they could contact her day or night to ask questions about the medication or their child's condition. Today, she regularly gets text updates about how the trial's graduates are starting new years at school, reaching milestone birthdays and celebrating holidays with their families.
As a co-author on the clinical trial results paper, Ireland hopes the peer-reviewed data will help speed up insurance approvals for use of the chemotherapy drugs in PVS and influence other cardiology departments to begin chemotherapy as soon as a child is diagnosed with progressive PVS.
Shifting pulmonary vein stenosis from acute to chronic
Despite the success of the trial, new challenges still lie ahead for the growing cohort of PVS survivors.
"The newly-formed 'chronic' PVS patient population is growing up with unique heart and lung conditions that require innovative treatment," Jenkins says. "For example, some children who did not receive chemotherapy early enough are now undergoing heart and lung surgeries to address scarring caused by PVS. Without chemotherapy, however, these children may not still be alive to face these medical challenges."
Although the number of patients with chronic PVS is still small, it's quickly growing. As each child reaches new milestones that were not possible before, Jenkins and Ireland adapt to create personalized treatment plans for each and every one of them.
"We tell parents that even though there haven't been many patients in your child's shoes, we've been the team that's seen them all," Ireland says. "There isn't another clinic out there that knows how to treat PVS like we do."
Original publication
Callahan R, Kieran MW, Baird CW, Colan SD, Gauvreau K, Ireland CM, Marshall AC, Sena LM, Vargas SO, Jenkins KJ; "Adjunct Targeted Biologic Inhibition Agents to Treat Aggressive Multivessel Intraluminal Pediatric Pulmonary Vein Stenosis"; J Pediatr.; 2018