Computer-Designed Customized Regenerative Heart Valves
Cardiovascular tissue engineering aims to treat heart disease with prostheses that grow and regenerate. Now, researchers from the University of Zurich, the Technical University Eindhoven and the Charité Berlin have successfully implanted regenerative heart valves, designed with the aid of computer simulations, into sheep for the first time.
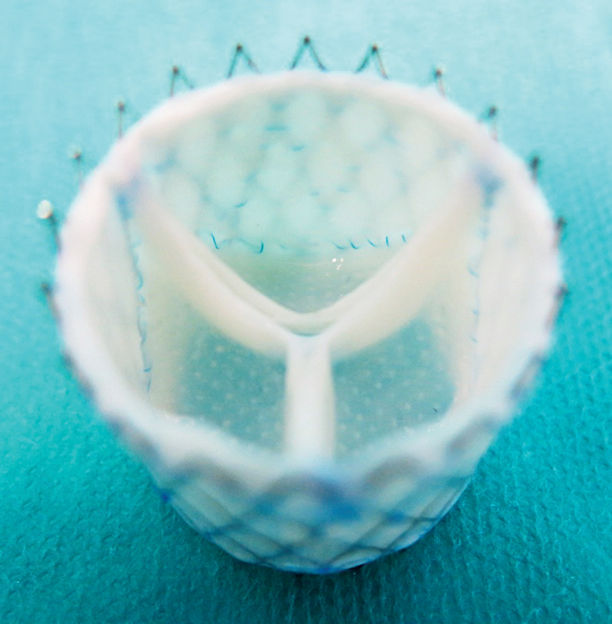
Computer-designed customized regenerative heart valve.
Image: adapted from figure in publication
Producing living tissue or organs based on human cells is one of the main research fields in regenerative medicine. Tissue engineering, which involves growing replacement parts in the laboratory, forms a key part of this research. The parts can be used to replace defective cells and tissues in the body and restore their normal functioning. The bioengineered replacements have significant advantages over the artificial implants currently in use: They do not cause immune reactions in the patient’s body, and they can grow and regenerate themselves.
Designing heart valves on the computer
An international consortium led by UZH Professor Simon P. Hoerstrup has now reached a milestone on the road towards being able to treat heart patients using new heart valves cultured from human cells: As part of the EU-funded project LifeValve, for the first time the team, using computer simulations, succeeded in individually predicting how well cultured heart valves would grow, regenerate, and function in large animal models (sheep). “Thanks to the simulations, we can optimize the design and composition of the regenerative heart valves and develop customized implants for use in therapy,” says Hoerstrup of the Institute of Regenerative Medicine at UZH.
Predicting regeneration – an important step towards clinical application
In particular, changes to the structure of the heart valve that occur in the body during the dynamic regeneration process can be predicted by computer simulations and anticipated accordingly in the design. The results that have now been published in the journal Science Translational Medicine are a significant step towards the routine application of the Zurich-developed tissue engineering technology in the future. Moreover, the findings provide a basic conceptual contribution that will aid the successful transfer to clinical use of new bioengineering technologies in regenerative medicine.
Current prostheses have to be regularly replaced in children
Valvular heart disease is one of the major causes of morbidity and mortality worldwide. Currently available artificial heart valve prostheses are an unsatisfactory solution, in particular for children with congenital heart defects. Children with defective heart valves or blood vessels often have to undergo an operation to have them replaced by prostheses which cannot grow as the child’s body grows. This means they then require multiple reoperations with an associated increased risk of surgical complications and considerable psycho-social stress for the young patients and their families. Prostheses of animal origin – e.g. from pigs or cows – also wear out with time and have to be replaced. Adult patients could therefore also benefit from regenerative heart valves and blood vessels.
No one-size-fits-all solution
While this field of research is promising and the first clinical uses of heart valves and blood vessels cultured using tissue engineering have already been made, there are still a few hurdles to get over before the technology can be routinely used. “One of the biggest challenges for complex implants such as heart valves is that each patient’s potential for regeneration is different. There is therefore no one-size-fits-all solution”, emphasizes Hoerstrup, whose research team has been among the pioneers of cardiovascular tissue engineering for more than 20 years.
The University Children’s Hospital Zurich is currently preparing a study treating children who have congenital heart defects with tissue engineered blood vessels, developed as part of the LifeMatrix project by Wyss Zurich. Wyss Zurich is a new center run jointly by the University of Zurich and ETH to support the clinical translation of innovative findings into novel medical therapies in the areas of regenerative medicine and robotics.
Original publication
Maximilian Y. Emmert et al.; "Computational modeling guides tissue-engineered heart valve design for long-term in vivo performance in a translational sheep model"; Science Translational Medicine. May 9, 2018
























































