A new formula for creating chemical reactions -- with carbs
New approach to glycosylation that is remarkably simple and works in water at room temperature
In the world of chemistry, good things can happen if you just add sugar.
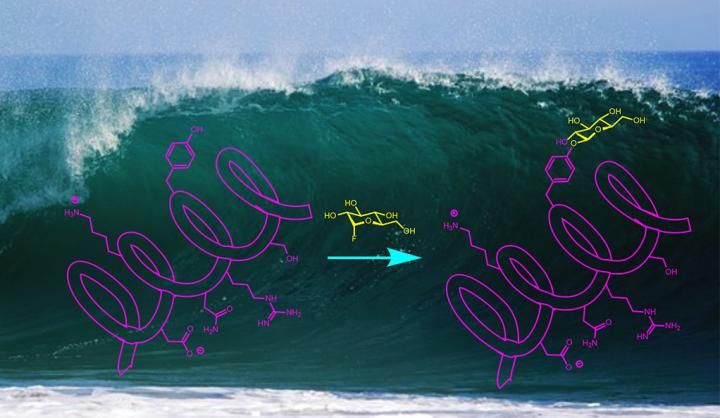
Yale University scientists have developed a new approach to glycosylation that works in water.
Yale University
A wide range of drugs and biochemical probes -- everything from antibiotics to Alzheimer's disease biomarkers -- rely on natural or synthetic compounds that aid a reaction by adding carbohydrates. It's a process called glycosylation. But it is traditionally a highly specific process that makes synthesis of such compounds, for testing or large-scale production, difficult.
A team of Yale University scientists has developed a new approach to glycosylation that is remarkably simple and works in water at room temperature. A study describing the process appeared April 30 in the online edition of Nature Chemistry.
"Glycoproteins serve a myriad of functions in biological chemistry," said Scott Miller, the Irénée du Pont Professor of Chemistry at Yale and co-corresponding author of the study. "Synthesis of these compounds is very challenging, and has limited the extent to which people can make multiple variants in order to find the best biochemical probes and therapeutics."
Alanna Schepartz, Sterling Professor of Chemistry at Yale and the study's other co-corresponding author, points out that many glycoprotein molecules are currently made using an enzyme catalyst. The Yale team's approach does not require an enzymatic reaction, allowing the process to be generalized to create large numbers of different compounds.
Another advantage of the new process, the researchers said, is that it occurs in water as the solvent. Often, organic synthesis of bioactive compounds happens in non-aqueous solvents, because water has a tendency to react with many catalysts and chemical agents.
"There is enormous interest right now in strategies to perform selective chemistry on complex biomolecules in water and also in cells," Schepartz said. "This paper lays a strong foundation that we and others can build upon to develop novel and useful chemistry."
The first author of the study is Tyler Wadzinski, a graduate student in Miller's lab. Co-authors, all of Yale, are Angela Steinauer, Liana Hie, and Guillaume Pelletier.
"This is truly a collaborative study that blends expertise in chemical reaction development and biological chemistry," Miller said.
Most read news
Topics
Organizations
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Nutrigenomics
What is it about your face?
VIB GM poplar field gets authorized
Rules_of_surgery
Leigh's_disease
Evotec expands neuroscience collaboration with Bristol Myers Squibb to include novel cell type - Triggers US$ 9.0 m payment to Evotec
Halcygen to acquire wholly owned subsidiary of Hospira
Finding a way forward in the fight against prion disease
List_of_world_bumblebee_species
Dun_gene























































