ADP-ribosylation on the right track
Scientists changing a 50-year-old paradigm
Scientific achievements enlarge our knowledge about how things work and eventually enable us to understand details and even to predict the unknown. But sometimes the assumptions we make based on what we have already seen limit our perception and bias our approach to the new. This is exactly what happened with ADP ribosylation (ADPr), a particular protein modification that appears in every cell and is essential for almost all biological processes.
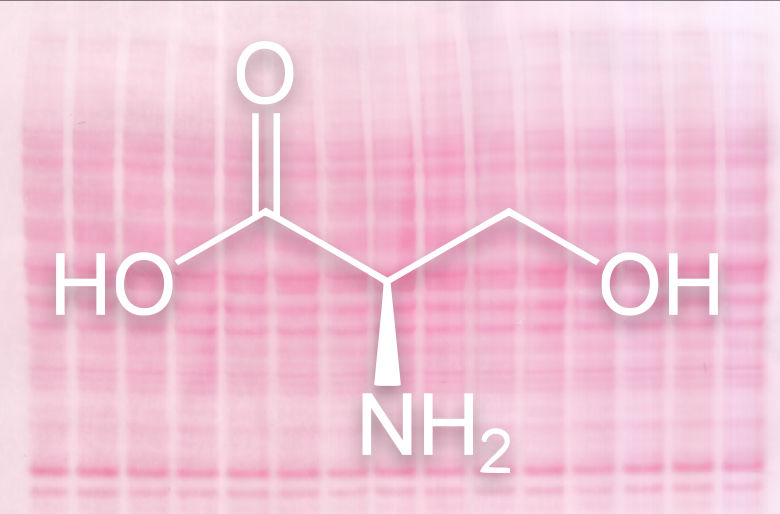
Chemical structure of the amino acid serine, which is part of almost all proteins and the main target for ADP ribosylation. In the background a membrane with stained proteins.
©Max-Planck-Institute für Biologie des Alterns
When a protein gets ADP ribosylated, it gets labeled with additional information. For example, an ADPr signal can be placed on a protein for the recruitment of vital factors to repair damaged DNA, the genetic information in cells. Like an alarm in case of an emergency, it marks the place where help is needed.
For half a century it was believed that ADPr modifies particular sites on proteins: the amino acids glutamate, aspartate, arginine and lysine. But the functional characterization of these identified sites showed very slow progress. “We know the reason for this now: most of the sites were mis-localised” says Orsolya Leidecker, a scientist in the group of Dr. Ivan Matić from the Max Planck Institute for Biology of Ageing. Now the scientists have finally identified the amino acid serine as the major site of ADPr by using a new technique. Due to its chemical structure, serine was never really considered as a target, which makes this finding all the more exciting.
“It’s a little like the discovery of the structure of the DNA”, explains group leader Dr. Ivan Matić. “People had known for decades that there must be genetic information stored somewhere but didn’t know where or how. The field of ADPr modification was similarly slow to develop for lack of precise knowledge about which amino acid ADPr attaches to. Now we finally know exactly where this information sits.”
Additionally, the Matić group and their collaborators in Oxford have developed a simple method for validating serine ADP-ribosylated sites in cells, which enables any scientist to examine ADPr on their protein of interest. “Anybody in any lab can perform the experiment and investigate if the modification of their protein is on serine” says Leidecker, who contributed to the main part of the work.
Actually, the identification of the correct position of ADPr is only the beginning. Researchers can now investigate the impact of ADPr on proteins, understand their functionality and develop strategies to use this modification as a target for drug development. Targeting processes regulated by ADPr is already a very promising strategy in treatment of cancer and acute cardiovascular conditions.
Original publication
Luca Palazzo, Orsolya Leidecker, Evgeniia Prokhorova, Helen Dauben, Ivan Matić, Ivan Ahel; "Serine is the major residue for ADP-ribosylation upon DNA damage"; eLife 2018;7:e34334
Most read news
Original publication
Luca Palazzo, Orsolya Leidecker, Evgeniia Prokhorova, Helen Dauben, Ivan Matić, Ivan Ahel; "Serine is the major residue for ADP-ribosylation upon DNA damage"; eLife 2018;7:e34334
Organizations
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.





















































