Brain cell's Achilles' heel may prompt hydrocephalus
Advertisement
viruses may spark hydrocephalus by exploiting a suprising weakness of cells that circulate fluid in the brain, says a new study by Duke University scientists.
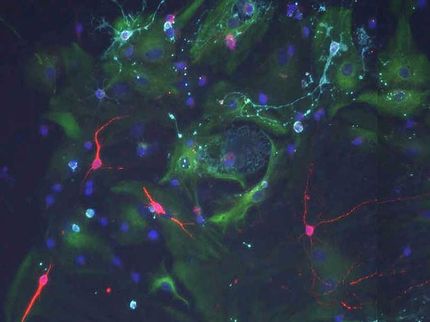
Brain cells shaped like sea anemones rapidly beat their arms to keep cerebrospinal fluid circulating.
Chay Kuo, Duke University
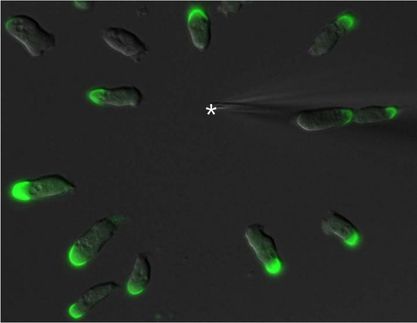
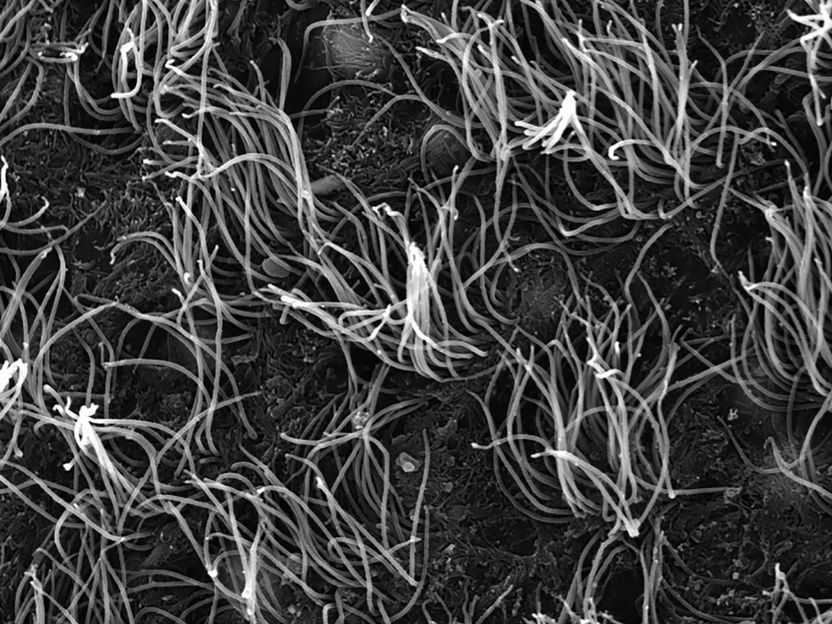
Cells shaped like sea anemones line the cavities of the brain, rapidly beating their cilia to keep cerebrospinal fluid circulating. These cells, called ependymal cells, provide homes to neural stem cells and can play a key role in preventing hydrocephalus, the potentially deadly build-up of cerebrospinal fluid in the brain.
The researchers discovered that mature ependymal cells from mice require a continuous production of a transcription factor called Foxj1 to maintain their shape and function. Without Foxj1, the cells lose their waving hair-like structures and rapidly revert to an earlier stage of development.
Viruses known to infect the brain have found a way to shut down the production of Foxj1 and disable the cells, the researchers report.
"We found that when we infected ependymal cells with viruses that can cause major problems in the human brain, ependymal cells completely degraded Foxj1 and transformed," said Chay Kuo, M.D., Ph.D., an associate professor of cell biology in the Duke School of Medicine. "They lost their cilia so they could not move fluid."
Currently the only treatment for hydrocephalus is inserting a shunt to drain excess cerebrospinal fluid, an approach which only works in roughly half of patients and can cause serious complications. This study indicates that drugs that preserve the production of Foxj1 in ependymal cells may provide an alternative treatment.
Kuo and postdoctoral fellow Khadar Abdi, Ph.D., initially set out to grow ependymal cells in hopes of studying their role in nurturing neural stem cells, Kuo said.
They found that infusing brain cell progenitors with a drug known to pump up the production of Foxj1 triggered more cells to grow into ependymal cells. Surprisingly, when they switched off the Foxj1 gene in mature ependymal cells in mice, the cells swiftly transformed back into their early undifferentiated state.
"This was quite shocking to us," Kuo said. "Many people thought that ependymal cells were terminally differentiated -- that when you are born, these are supposed to last a lifetime."
In another unexpected experimental result, Abdi observed that Foxj1 is fragile: the protein can degrade in as little as two hours, meaning that cells must continuously manufacture new Foxj1 to preserve their cilia and shape.
"They are constantly running to stand still," Kuo said.
The team discovered that ependymal cells use an enzyme called IKK2 to spur the production of Foxj1. A number of viruses, including the herpes simplex virus, have evolved machinery to block IKK2 and, in the process, halt the continuous production of Foxj1.
Wellington Cardoso, M.D., professor of medicine and director of the Columbia Center for Human Development at the Columbia University Medical Center, praises the study for charting how multiciliated ependymal cells maintain their identity and function in the brain, thus preventing brain defects such as hydrocephalus.
"This study not only answers the question of whether these ependymal cells are stable or plastic, but also provides the mechanism that makes them lose their features and turn into something no longer functional," said Cardoso, who was not involved in the study. "The paper nicely identifies and links all the little pieces of this mechanism. It is like reconstructing the scene of a crime where they identify every culprit."
"I would be curious to see if this same mechanism applies for other multiciliated cells of the body, like those in the airways of the respiratory tract," Cardoso said.
For his part, Kuo wants to know why an important cell in the brain would have such an unexpected Achilles' heel.
"There are a class of viruses that have figured out that ependymal cells have this major weakness, so why hasn't evolution eliminated this back door for viruses to target the brain?" Kuo said. "There must be a benefit."