Diamond ring architecture of a protein complex
Just as diamond ring is needed in marriage, NuA4/Tip60, a complex with diamond ring architecture, is required for regulatory and repairing processes. Prof. CAI Gang and Prof. Jacques Côté's team reports the 4.7 Å structure of the yeast NuA4/TIP60 complex, which elucidates the detailed architecture and molecular interactions between NuA4 subunits.
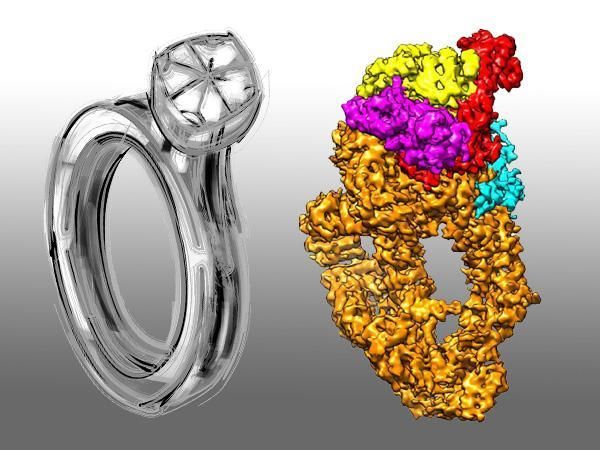
The NuA4 cryo-EM structure is akin to a diamond ring.
USTC News Center
NuA4/Tip60 is a complex which catalyzes diverse substrates critical for gene regulation, DNA repair, and cell cycle progression. Yet its compositional complexity and conformational flexibility have long impeded researchers from exploring at high precision. By utilizing cryo-electron microscopy (cryo-EM), CAI Gang's team provides a high-resolution view of this complex in sub-nanometer scale, and discloses how NuA4/Tip60 assemblies for the first time.
The 4.7 Å resolution structure illuminates Tra1/TRRAP and Eaf1 serve as a scaffold for NuA4/TIP60 assembly. Although Tra1/TRRAP lacks of kinase catalytic activity, it adopts active conformation of the catalytic domain in NuA4/TIP60 assembly. Besides providing more insights about scaffolding and regulatory mechanisms of Tra1/TRRAP, the structure elucidates more details about NuA4/TIP60 subunits.
Unexpectedly, the structure also shows human TRRAP mutations are largely centered on the Tra1/TRRAP interaction surfaces mediating NuA4/TIP60 assembly. Since Tra1/TRRAP contains hotspots for tumorous generation, this observation warrants preventing cancer by targeting the scaffolding function of TRRAP. In addition, since Tip60 is significantly down-regulated in many cancers (i.e. breast and prostate), specific inhibitors of Tip60 will promise major breakthrough in cancer treatment.
Talking about future plans, CAI Gang will focus on obtaining structure of the holoenzyme and determining the substrate specificity and catalytic mechanism of NuA4/Tip60. These could greatly facilitate the development of specific TIP60 inhibitors and potent chemotherapeutic drugs, aiming at treating more people suffering from cancer.
Original publication
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Decomposition of textile products in the ground - Laboratory tests help with evaluation of environmental compatibility
Nutrigenomics
What is it about your face?
VIB GM poplar field gets authorized
Rules_of_surgery
Leigh's_disease
Evotec expands neuroscience collaboration with Bristol Myers Squibb to include novel cell type - Triggers US$ 9.0 m payment to Evotec
Halcygen to acquire wholly owned subsidiary of Hospira
Finding a way forward in the fight against prion disease
List_of_world_bumblebee_species
Dun_gene






















































