Near-infrared photoactivatable oxygenation catalysts of amyloid peptide
A new, biocompatible photooxygenation catalyst that can selectively oxygenate and degrade the pathogenic aggregation of Alzheimer's disease (AD)-related amyloid-β peptide (Aβ) under near-infrared (NIR) light irradiation is developed. The catalyst was able to oxygenate Aβ embedded under the skin of a living mouse, and diminished intact Aβ level in AD-model mouse brain. The new catalyst is potentially applicable for the treatment of peripheral amyloid diseases and AD.
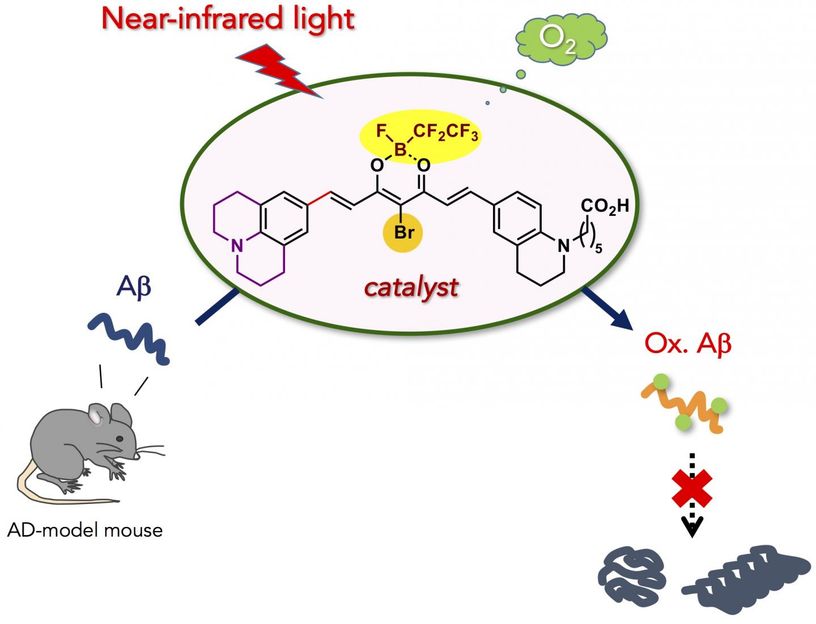
Near-infrared light-activatable oxygenation catalyst of amyloid-β (Aβ) was developed. Catalytic photooxygenation under the mouse skin was possible. Moreover, application of photooxygenation catalyst diminished the intact Aβ in the mouse brain.
The University of Tokyo
Toxic aggregation of amyloid peptide and protein is intimately related to a number of human diseases. Amyloid-β (Aβ) is a representative amyloid peptide whose aggregation is related to the pathogenesis of Alzheimer's disease (AD). The development of an artificial chemical system that selectively converts toxic amyloid aggregates to non-toxic species under physiologic conditions, thereby potentially suppressing the pathogenic process, could be a novel therapeutic strategy to treat currently-incurable amyloid diseases, including AD.
We envisioned that photocatalyzed aerobic oxygenation would be a suitable chemical reaction to attenuate the pathogenic aggregative properties of Aβ under physiologic conditions. Because peptide and protein aggregation generally depends on intermolecular hydrophobic interactions, covalent installation of hydrophilic oxygen atoms to a peptide or protein (i.e., oxygenation) would decrease the aggregative property. We previously reported that aerobic oxygenation of Aβ proceeds in the presence of Flavin (vitamin B2)-based photocatalysts, and the resulting oxygenated Aβ exhibits very low aggregative ability and toxicity. Thereafter, more selective photooxygenation catalysts, activated only when sensing a toxic higher-order amyloid structure, were developed based on a fluorescence probe for aggregated amyloid peptide and protein. In vivo application of the catalysts was, however, unfeasible, because irradiation with visible light, which has low tissue penetration, was necessary for catalyst excitation. For in vivo application, photocatalysts must be able to function under excitation with longer wavelength light, referred to as the "optical window" in which living tissue absorbs relatively little light.
Here, we developed biocompatible photooxygenation catalyst that can selectively oxygenate and degrade the pathogenic aggregation of Aβ under near-infrared (NIR) light irradiation. The catalyst exhibited four main advantages compared with the previous catalysts for degrading aggregated and toxic Aβ:
- High selectivity for aggregated Aβ that stems from the higher-order amyloid structure-sensing on/off switch for the catalyst activity. The precise target selectivity allowed for photooxygenation of aggregated Aβ in the presence of the cells and in mouse brain lysate.
- Low toxicity to the cells. Structural optimization of the catalyst markedly reduced the cytotoxicity, both in the dark and under NIR irradiation.
- High oxygenation potency under NIR photoirradiation. Due to tissue-permeability of NIR light, photooxygenation of aggregated Aβ under the mouse skin was possible in high yield.
- Applicability to the living animal brain in vivo. Injection of the catalyst to the AD-model mouse brain along with NIR light irradiation led to significant decrease of the intact Aβ level in the brain. The results obtained in this study are an important step to using artificial catalysis as a potential therapeutic strategy against amyloid diseas
Original publication
Most read news
Original publication
Jizhi Ni and Atsuhiko Taniguchi and Shuta Ozawa and Yukiko Hori and Yoichiro Kuninobu and Takashi Saito and Takaomi C. Saido and Taisuke Tomita and Youhei Sohma and Motomu Kanai; "Near-Infrared Photoactivatable Oxygenation Catalysts of Amyloid Peptide"; Chem; 2018
Topics
Organizations
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents

Microplastics in freshwaters - The risks to the environment are (still) low

‘Dramatic and rapid’ regression of glioblastoma after next generation CAR-T therapy - Researchers took a new approach to CAR-T, engineering CAR-TEAM cells to treat mixed cell populations within tumors

Evolution in a test tube: these bacteria survive on deadly copper surfaces - Tiny survivalists in the lab produced and more closely studied
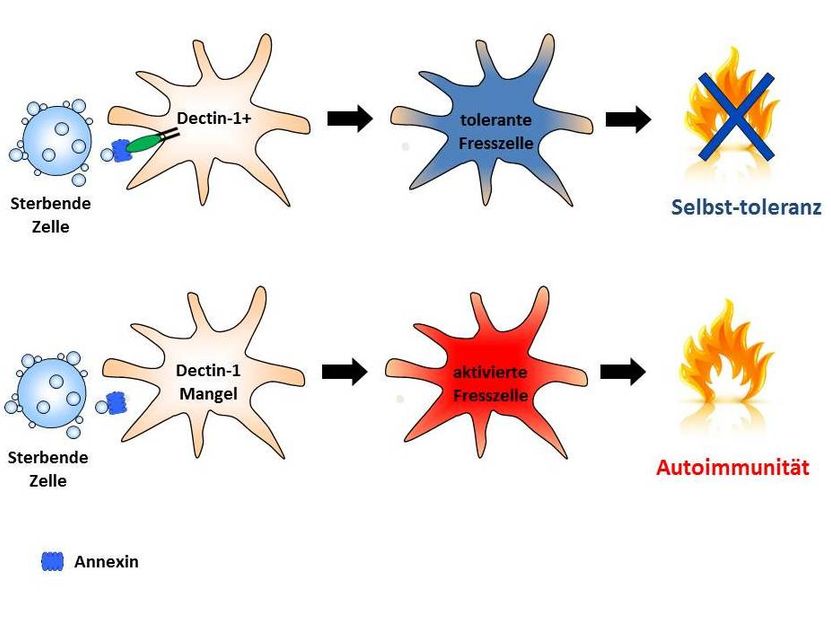
How dying cells prevent dangerous immune reactions
Medicinal_plants_of_the_American_West

R&D Systems Inc. - Minneapolis, USA
Astasia-abasia

Evolution of ebola virus resulted in increased human infectivity

Biofrontera transfers clinical research to the U.S. - "We can be much more flexible in our strategy to expand our market presence in Europe thanks to the newly defined cost structure"
F-star and Boehringer Ingelheim to collaborate in discovery and development of therapeutic antibodies
German and Israeli scientists gain new insights into protein disposal