Potential for a green energy economy based on hydrogen
First Characterization of a Sensory [FeFe] Hydrogenase
hydrogenases are enzymes capable of making hydrogen gas (H2) using protons from water, a reaction with relevance to a potential future green energy economy based on H2.
Max-Planck-Institut für Chemische Energiekonversion
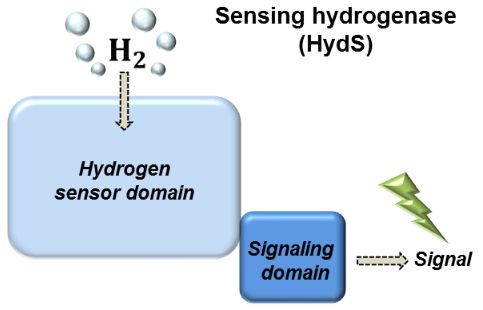
Bacteria containing these enzymes often produce H2 as a waste product during sugar metabolism in the absence of oxygen. Meanwhile, other types of bacteria consume this H2 as an energy source. Hydrogenases, the key enzymes in both these processes, are only required under specific conditions, and so their synthesis inside the bacterium must be regulated in response to the amount of H2 present. This regulation is achieved using sensory hydrogenases capable of sensing and signaling the concentration of H2 to the bacterial protein synthesis machinery.
One particular class of sensory hydrogenases, the so-called sensory [FeFe] hydrogenases (HydS), have remained completely uncharacterized so far. Now, a team from the Max Planck Institute for Chemical Energy Conversion in Mülheim an der Ruhr in Germany together with the Institute of Low Temperature Science at the University of Hokkaido in Japan, have produced and characterized a HydS enzyme from the thermophilic bacterium Thermotoga maritima. Using the recently developed technique of artificial maturation together with advanced spectroscopic methods, the researchers showed that the protein environment of the catalytic center is elegantly tuned to optimize this protein for its sensory function.
The researchers demonstrate that the catalytic center is very sensitive to low amounts of H2 allowing the enzyme to effectively signal the presence of H2 to the regulatory system of the bacterium. This research provides a key step forward for our understanding of how these sensory enzymes work. Furthermore, knowing which adaptations tailor the sensory enzymes for their function tells us about how the other [FeFe] hydrogenases that produce hydrogen work.
Ultimately, a complete picture of the enzyme mechanism may provide important clues for telling chemists how to build better catalysts for water electrolyzers and hydrogen fuel cells, allowing the dream of a future hydrogen energy economy to become a reality.
Original publication
Most read news
Original publication
Nipa Chongdar, James A. Birrell, Krzysztof Pawlak, Constanze Sommer, Edward J. Reijerse, Olaf Rüdiger, Wolfgang Lubitz, Hideaki Ogata; "Unique spectroscopic properties of the H-cluster in a putative sensory [FeFe] hydrogenase"; Journal of the American Chemical Society; 2017
Organizations
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.