Merck Reports Results of Phase III Study of Investigational Oral Allergy Immunotherapy Tablet (AIT)
In new data from a Phase III study in 345 children and adolescents (ages 5-17 years), patients with grass pollen allergic rhinoconjunctivitis treated with Merck's investigational sublingual grass (Phleum Pratense) allergy immunotherapy tablet (AIT) showed a 26 percent greater improvement in the total combined score (daily symptom score and daily medication score), compared to patients receiving placebo (p=0.001). Allergic rhinoconjunctivitis, or runny nose and itchy, watery eyes due to allergies, is a common condition in children and adolescents. These data were presented at the American Academy of Allergy, asthma & Immunology Annual Meeting in New Orleans.
AIT is an investigational, dissolvable oral tablet that is designed to prevent allergy symptoms by inducing a protective immune response against allergies, thereby treating the underlying cause of the disease. Merck is investigating AIT for the treatment of grass pollen allergic rhinoconjunctivitis in both children and adults. AIT is currently approved in the European Union (EU) for children and adults (ages 5-65) with grass pollen allergy, marketed under the name GRAZAX® by ALK-Abello, which discovered and developed the product in the EU.
“Patients who received AIT experienced significant reductions in allergy symptoms and allergy medication use, the combined symptom scores evaluated in the study,” said Michael Blaiss, M.D., clinical professor of pediatrics and medicine, University of Tennessee Health Science Center in Memphis, Tenn., and lead investigator for the study. “This Phase III study is the first to evaluate AIT in children and adolescents with grass pollen allergies in North America.”
The randomized, placebo-controlled, Phase III study investigated the efficacy and safety of AIT versus placebo in the treatment of allergic rhinoconjunctivitis caused by pollen from Timothy grass, a type of grass common in North America. A total of 345 patients, ages 5-17 years, were randomized to daily treatment with oral grass AIT (15mcg of Phl p5, which is a formulation of the timothy grass allergen) or placebo at least eight weeks prior to and throughout the 2009 grass pollen season. The primary efficacy endpoint was the total combined score, comprised of total daily symptom score and total daily medication score. Adverse events experienced by patients receiving AIT were generally local, application site reactions and included oral pruritus, throat irritation, stomatitis, ear pruritus, mouth edema, oropharyngeal pain, pharyngeal erythema, eye pruritus and lip swelling. There were no reports of anaphylactic shock in the study. Eighty-nine percent of study patients were sensitive to allergens in addition to the grass pollen allergen.
Most read news
Organizations
Other news from the department research and development

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
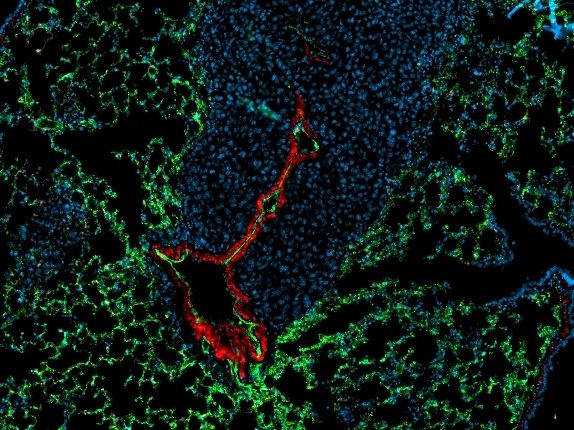
Preventing metastasis - an antibody with therapeutic potential - New principle for slowing down the metastatic dissemination of cancer cells
Multi-organ platform for risk assessment of nanomaterials - Multimodular microchip platform for predicting the behaviour of nanomaterials in the body
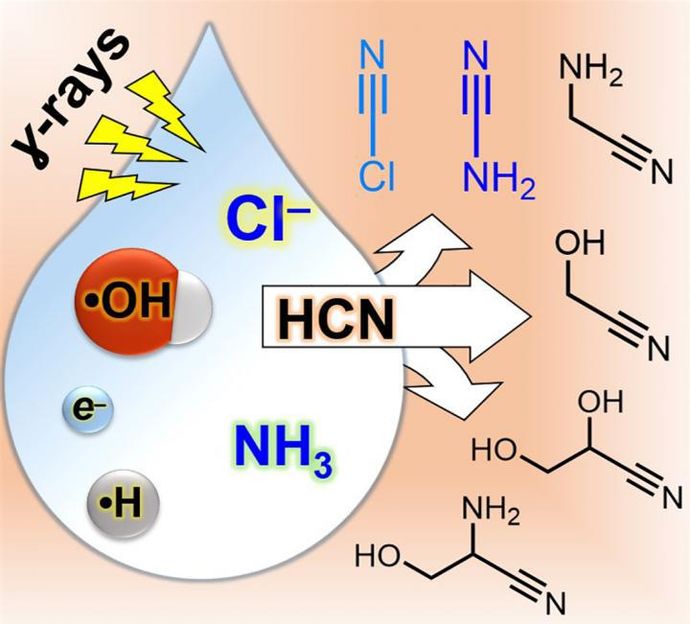
Common table salt may have been crucial for the origins of life
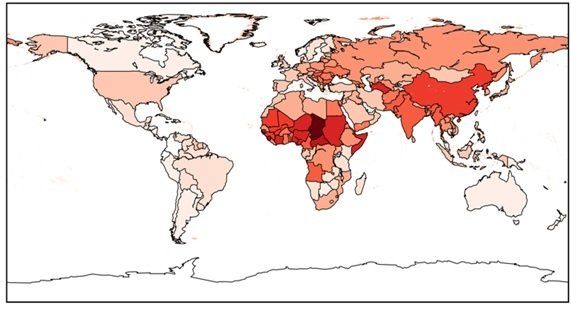
Air pollution is one of the world's most dangerous health risks - The effects of air pollution shorten the lives of people by an average of almost three years
Chinese_herbology

Fine particulate matter catalyzes oxidative stress in the lungs - Study sheds new light on the adverse health effects of air pollution

MiDiagnostics secures €30 million Series D funding round led by Thermo Fisher Scientific - Innovative sterility and other quality control tests for use in the BioPharma Industry

Grünenthal acquires US-company Valinor Pharma - Valinor Pharma Announces Acquisition by Grünenthal with a Total Deal Value of Approximately $250 Million
GSK and Genmab Announce Results from Trial of Arzerra in Fludarabine and Alemtuzumab Refractory Chronic Lymphocytic Leukemia
De_humani_corporis_fabrica
Oral antibiotics may raise risk of kidney stones






















































