Pharming's C1 inhibitor product potentially effective in reducing complications following transplantation
Pharming Group NV announced publication of preclinical evidence that its recombinant human C1 inhibitor (rhC1INH) may play an important therapeutic role in the prevention of delayed graft function (DGF) after solid organ transplantation. The published study results further indicate the potential for the product in the treatment of ischemia-reperfusion injuries and in particular in the prevention of DGF following kidney transplantation.
Pharming's rhC1INH significantly reduced several ischemia/reperfusion-related inflammatory processes and significantly limited tissue damage in a swine model of ischemia/reperfusion-induced renal damage. As the inflammatory processes in pigs are very similar to those in humans, these results form a clear indication of the potential of rhC1INH for treatment and prevention of ischemia tissue damage in patients.
Ischemia/reperfusion injury is the major cause of DGF in transplanted kidneys. DGF is an early sign associated with poor long-term graft function and survival. Current treatments of DGF include the use of immune suppressing agents. Much attention is also paid to prevention of DGF by continuous improvement of the transplantation procedure itself and the conditions under which the organs or tissues are stored and treated during the procedure. Because of its unique anti-inflammatory properties Pharming's rhC1Inh may complement current transplantation procedures and current treatment of DGF.
In October 2009, Pharming announced the beneficial results of another preclinical study in the field of ischemia-reperfusion injuries, focussing on the reduction of damage following brain infarcts (stroke). Several studies in rodents indicate that the complement system plays a pivotal role in renal ischemia reperfusion injury. To date, however, limited information was available from humans and larger animals. This swine study has been supported by a research grant from Pharming and was performed by researchers from the University of Bari, Italy. The results are published in the American Journal of Pathology Feb 2010; 176(4) by Dr Giuseppe Castellano and Professor Giuseppe Grandaliano from the University of Bari.
Topics
Organizations
Other news from the department research and development

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
More news from our other portals
Last viewed contents

Natural molecule could improve Parkinson's

Laboratory study: effect of antibodies against omicron variants BA.1 and BA.2 wears off quickly

Covid-19 can trigger diabetes - When SARS-CoV-2 infects beta cells, they produce less insulin and show signs of death
Spinifex to be acquired by Novartis - Australian pain drug innovation company sold to Novartis for US$200m plus milestone payments
New mechanism that leads to inflammation in rheumatoid arthritis discovered
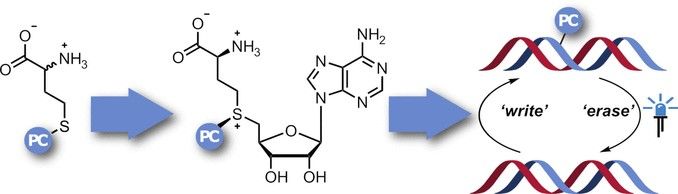
Defined Blockade: Enzymatic photocaging for the study of gene regulation through DNA methylation
Category:Voice_disorders
Fusarium_oxysporum
Category:Indole_alkaloids

Scientists develop novel nanoparticles that could serve as contrast agents - Special feature: The properties of these unique nanoparticles change in reaction to heat