Compounds that help protect nerve cells discovered by Duke team
Advertisement
Scientists at Duke University Medical Center have found some compounds that improve a cell's ability to properly "fold" proteins and could lead to promising drugs for degenerative nerve diseases, including Huntington's disease, Alzheimer's disease and Parkinson's disease. The research was published in PLoS biology.
Misfolded proteins in nerve cells (neurons) are a common factor in all of these diseases. The Duke team has identified many new chemicals that activate a master regulator to increase the supply of "protein chaperone" molecules that help fold proteins properly.
The scientists further explored one of the candidate molecules to activate the master regulator of chaperone gene expression, Heat Shock Factor 1 (HSF1), to learn whether it would work in model systems of Huntington's disease, a devastating neurodegenerative disease of protein misfolding.
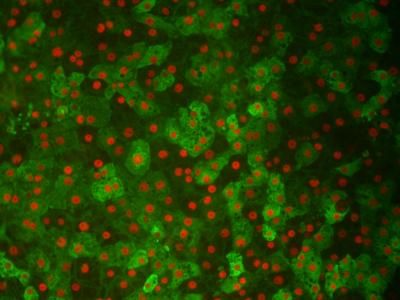
They were able to show that the molecule stimulated protein chaperones in cells and in an animal system. The damage to early-state rat neurons was much lower in cells pre-treated with the HSF1 activator, and damage to the neurons of fruit flies that had a Huntington's-like disorder was also greatly reduced.
Previous studies suggested that elevating the abundance of protein chaperones is effective in treating cell and animal models of Huntington's and Parkinson's diseases. This work provides a new approach to address the root cause of these diseases -- protein misfolding. Earlier attempts had used heat shock and other approaches that stress a nerve cell in order to produce more chaperone molecules, but at a cost of damaging the cell to save it.
"The advantage of our screen is that it identifies molecules that can elevate the levels of chaperones without inducing cellular stress and that don't inhibit a key protein chaperone called Hsp90 that is needed for cells to function normally," said senior author Dennis J. Thiele, Ph.D., Professor of Pharmacology and Cancer Biology. "We found a creative way to identify new molecules that can activate the body's natural protein folding machinery."
Lead author Daniel Neef, Ph.D., says they used genetically altered yeast to find compounds that might aid chaperone development. The scientists took yeast with a deleted HSF1 (master regulator) gene and inserted the related human HSF1 gene. These yeast, however, still weren't able to activate human HSF1 on their own, and in effect, died. They needed an additional molecule to make human HSF1 become active.
The team put these "humanized yeasts" into wells and started testing compounds that would provide the missing link. In several of the wells, if the compound worked, the yeast started multiplying. "Out of over 12,000 compounds tested from chemical libraries, about 50 compounds worked," Neef said. The team decided to explore one of these compounds (HSF1A) in further experiments.
"The humanized yeast-based screening results in our study provide a way to identify new classes of small molecules, small enough to penetrate the blood-brain barrier to work in neurons, in flies as well as in humans," Thiele said. "These small molecules may be effective therapies in neurodegenerative diseases caused by protein conformational disorders such as Huntington's, Alzheimer's and Parkinson's disease."
The scientists found that HSF1A could stimulate more protein chaperones and reduce the protein misfolding. They showed that adding a small amount of HSF1A to the developing rat neurons kept the proteins dissolved throughout the cell, rather than clumping visibly as speckled areas (as seen under microscopes).