Johns Hopkins researchers say vaccine appears to 'mop up' leukemia cells Gleevec leaves behind
Johns Hopkins Kimmel Cancer Center researchers say preliminary studies show that a vaccine made with leukemia cells may be able to reduce or eliminate the last remaining cancer cells in some chronic myeloid leukemia (CML) patients taking the drug Imatinib mesylate (Gleevec).
Gleevec, one of the first targeted cancer therapies with wide success in CML patients, destroys most leukemic cells in the body, but in most patients, some cancerous cells remain and are measurable with sensitive molecular tests. These remaining cells are a source of relapse, according to the investigators, especially if Gleevec therapy is stopped.
In a pilot study published in Clinical Cancer Research , the Johns Hopkins investigators used a vaccine made from CML cells irradiated to halt their cancerous potential and genetically altered to produce an immune system stimulator called GM-CSF. The treated cells also carry molecules, called antigens, specific to CML cells, which prime the immune system to recognize and kill circulating CML cells.
The study vaccine was given to 19 CML patients with measurable cancer cells, despite taking Gleevec for at least one year. A series of 10 skin injections were given every three weeks for a total of four times. After a median of 72 months of follow-up, the number of remaining cancer cells declined in 13 patients, 12 of whom reached their lowest levels of residual cancer cells. In seven patients, CML became completely undetectable. Because the study was conducted in a limited number of patients and not compared with other therapies, the researchers warn they cannot be sure that the responses were a result of the vaccine.
"We want to get rid of every last cancer cell in the body, and using cancer vaccines may be a good way to mop up residual disease," says Hyam Levitsky, M.D., professor of oncology, medicine and urology at the Johns Hopkins Kimmel Cancer Center. More research to confirm and expand the results is needed, Levitsky said.
The investigators will be testing blood samples taken from the study patients to identify the precise antigens that the immune system is recognizing. With this information, they will tailor their vaccine for additional studies that monitor immune response more precisely.
Patients receiving the trial vaccine experienced relatively few side effects that included injection site pain and swelling, occasional muscle aches and mild fevers.
Most read news
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Study pinpoints, prevents stress-induced drug relapse in rats
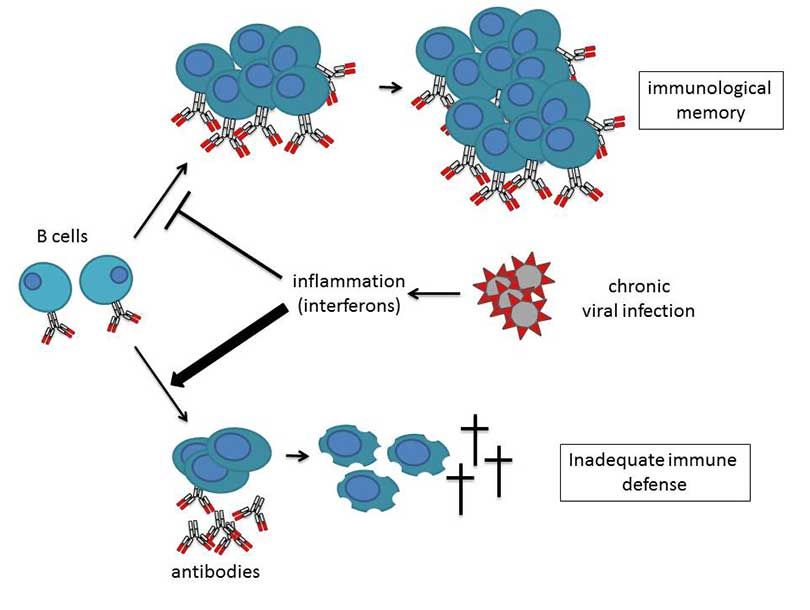
Inflammation Triggers Unsustainable Immune Response to Chronic Viral Infection
List_of_medical_abbreviations

New biotech venture PHIOGEN to tackle the global threat of antimicrobial resistance - By optimizing nature’s defenders, the team has produced unprecedented phage treatments
Epigenomics AG Successfully Validates Lung Cancer Test in Clinical Trial