Epigenomics AG: PRESEPT Study Subject Enrollment Successfully Completed
Completion of PRESEPT Study and reporting of top-line results expected for early 2010
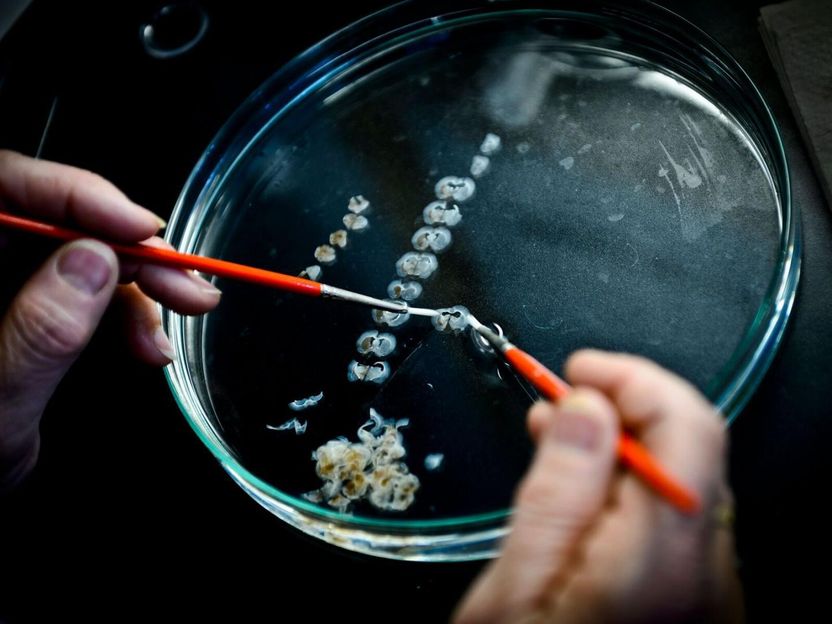
Epigenomics AG, sponsor of the PRESEPT Study reports that the enrollment of subjects was successfully completed. By December 16, total prospective enrollment has reached 7,852 study subjects at 32 clinical sites in the U.S.A. and Germany. In this representative screening population a total of 52 potential invasive colorectal adenocarcinoma cases have been identified by colonoscopy until that day exceeding the originally targeted number of 50 cases. To date 49 have been confirmed by pathological examination of tissue obtained by biopsy or surgical resection. Epigenomics expects the remaining colorectal cancer cases to be confirmed following scheduled surgeries during December 2009.
PRESEPT is a prospective multi-center clinical research study started in 2008 to evaluate the performance characteristics and health economic benefit of colorectal cancer screening using Epigenomics' Septin9 blood test in an asymptomatic screening population. Once completed, the PRESEPT Study will likely be the largest privately sponsored colorectal cancer screening studies ever conducted. The PRESEPT screening study follows the successful clinical validation of the Septin9 biomarker as an aid in the diagnosis of colorectal cancer in eight case control studies with in total more than 3,300 colorectal cancer patients and controls conducted by Epigenomics between 2005 and 2009.
With PRESEPT, Septin9 blood testing will be benchmarked against colonoscopy, the gold standard in colorectal cancer diagnosis, to determine the sensitivity and specificity of the Septin9 test for colorectal cancer and different classes of precancerous polyps (adenomas) when applied in its intended use population. Following a predefined statistical analysis plan a subset of about 1,500 of the PRESEPT blood plasma samples were independently selected and are being tested for Septin9. This subset includes blood samples from all confirmed invasive colorectal carcinoma cases, 55 cases with carcinomas in situ, a subset of cases with other advanced adenomas, approximately two hundred randomly selected cases with polyps less than 10 mm and a random selection of about one thousand colonoscopy-verified subjects with no apparent colon disease. The plasma samples to be tested were selected by an independent biostatistics team at the University of Minnesota and subject code and clinical status are masked to the testing laboratories. Following the Septin9 testing of the plasma samples, the test results are sent by the testing laboratories directly to the biostatistics team at the University of Minnesota, where the sample identities will be unmasked and the Septin9 testing data compared to the colonoscopy results and the histopathological findings.
The PRESEPT blood samples are tested in three independent high-profile laboratories, namely Quest Diagnostics Incorporated headquartered in Madison, NJ, U.S.A., ARUP Laboratories, Salt Lake City, UT, U.S.A. and the Institute of Laboratory Medicine and Pathobiochemistry of Charité - Universitätsmedizin Berlin, Germany. The laboratories use Epigenomics' recently launched CE-marked Epi proColon test kit to detect the Septin9 biomarker. Testing has been ongoing since October 2009 and is in the final stages of completion. After unmasking of the sample identities and data analysis, the Study Principal Investigator, Dr. Timothy Church, University of Minnesota, along with the independent PRESEPT Clinical Study Steering Committee, chaired by Prof. David Ransohoff, University of North Carolina, will report the results of the PRESEPT Study according to all applicable standards of scientific and clinical research.
Epigenomics expects to provide initial results in early 2010 demonstrating whether Septin9 testing using the Epi proColon assay meets the requirements put forth in current US colorectal cancer screening guidelines in detecting "the majority of prevalent or incident cancers at the time of testing". The detailed data analysis will subsequently be submitted to a top-tier journal for peer review and will be presented at major medical conferences beginning in the first half of 2010.
Most read news
Topics
Organizations
Other news from the department research and development
These products might interest you

Whatman™ folded filter papers by Cytiva
Whatman folded filter papers
Convenient folded formats speed up your sample preparation

Systec H-Series by Systec
Safe, reproducible and validatable sterilization of liquids, solids and waste
Autoclaves with 65-1580 liters usable space, flexibly expandable for various applications

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents

Tokyo Institute of Technology - Tokio, Japan
Neandertal gene variant increases risk of severe Covid-19 - A segment of DNA that causes their carriers to have an up to three times higher risk of developing severe Covid-19 is inherited from Neandertals

Point-of-care diagnostics: New methods for detecting single molecules - Pathogen-specific detection: Even one single DNA molecule is enough
Unstable chromosomes can promote breast cancer - Altered chromosomes lead to treatment resistance
Digestive_disease