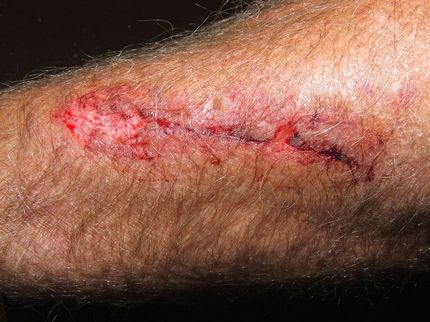
Polyheal Announces Positive Clinical Results of its Lead Product, "Polyheal-1", for the Treatment of Chronic Wound Healing
Polyheal Ltd. announced positive clinical trial results of its lead product "Polyheal -1" for the treatment of deep and chronic wounds. The trial is a randomized double blind controlled comparing "Polyheal-1" to standard of care therapy. The trial was conducted in five medical centers in Israel and included 66 patients who were treated for 4 weeks with either "Polyheal-1" or standard of care and followed for another 8 weeks with standard of care in both groups. The patients had suffered from these wounds 16 months on average, and had been treated previously with 2-3 previous treatment options on average.
Polyheal achieved the primary and secondary endpoints of the study. More Polyheal patients achieved coverage with red granulation tissue than the control (65% vs. 19%, p= 0.0006). Polyheal patients had greater 4 week surface area reduction (40% vs. 15%, p = 0.02) than control. The number of wounds that closed completely in the Polyheal group was twice that of the control group. No significant side effects were observed in connection with "Polyheal-1".
“This trial is unique in that it took deep complex ulcers that had failed to respond to available treatments and demonstrated improvement rates that we would expect to see in superficial ulcers. This is particularly important since it is precisely these complex wounds that put patients at greatest risk of complications such as infection and amputation,” says Prof Jan Apelqvist, at the Diabetic Foot Center in Malmö, Sweden and an international expert in the field of wound healing. “These encouraging preliminary clinical results put Polyheal among the most promising new wound healing technologies in an area with a substantial need of additional treatment strategies.”
Most read news
Organizations
Other news from the department research and development

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.