Genzyme Laboratory achieves accreditation from College of American Pathologists as first commercial genetic testing facility
Genzyme Corporation announced that its genetic testing laboratory located in Phoenix, Arizona, has obtained the CAP 15189SM accreditation to the International Standards Organization 15189:2007 Standard for laboratories from the College of American Pathologists. This accreditation recognizes the laboratory’s technical competence and continual quality management and underscores Genzyme’s commitment to improved patient outcomes by providing reliable test results.
The College of American Pathologists offers laboratories the opportunity to be measured against the quality standards outlined by the International Organization of Standardization. The College of American Pathologists rigorously assessed Genzyme’s quality management systems over the course of a full year before awarding it the CAP 15189 designation.
“Achieving this accreditation demonstrates our commitment to achieving superior quality standards and delivering overall operational excellence,” said Jon Hart, senior vice president and general manager of Genzyme Genetics, the business unit of Genzyme Corporation focused on complex testing services for pathology, oncology, reproductive and genetic medicine. “Going through the rigorous and extensive accreditation process has allowed us to mitigate risk and continually provide highly accurate test results to physicians and patients nationwide.”
Genzyme expects to pursue CAP 15189 accreditation in all of its genetic testing laboratories located throughout the United States.
Organizations
Other news from the department politics & laws
These products might interest you

Systec H-Series by Systec
Safe, reproducible and validatable sterilization of liquids, solids and waste
Autoclaves with 65-1580 liters usable space, flexibly expandable for various applications

Whatman™ folded filter papers by Cytiva
Whatman folded filter papers
Convenient folded formats speed up your sample preparation

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
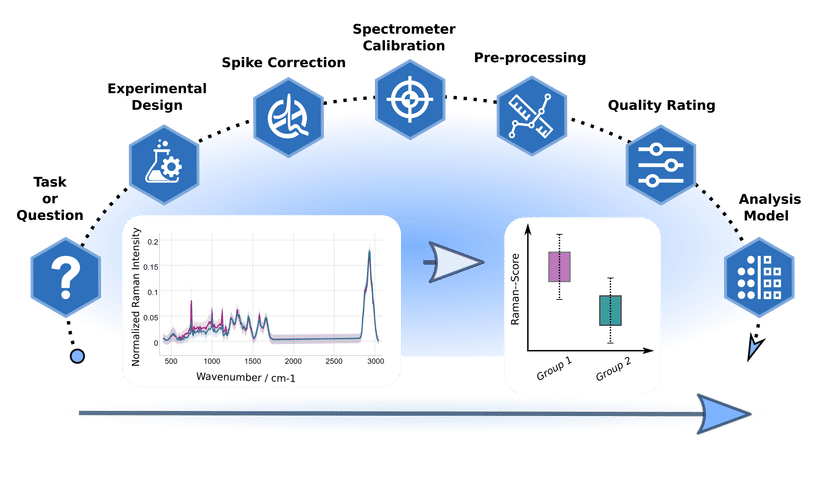
Artificial intelligence for better diagnostics - Standardized methods facilitate the evaluation of Raman spectra
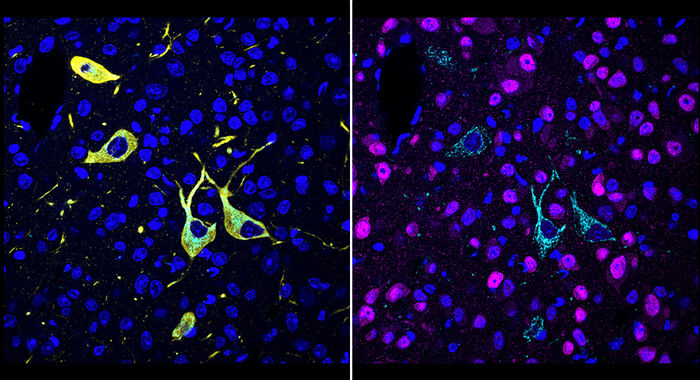
New technique shows in detail where drug molecules hit their targets in the body - The new method could speed the development of many new drugs
ChondroGene published paper ´Human genome project and cardiovascular disease genes´ - In landmark textbook ´Molecular Basis of Cardiovascular Disease´
CV Therapeutics Appoints Joseph M. Davie, M.D., Ph.D. to Board of Directors
ProtAffin AG appoints Dr. Simon Moroney to Supervisory Board
PerkinElmer Life Sciences Introduces Protein S-Nitrosylation Detection Technology - New NitroGlo(TM) Detection Kit Offers New Capabilities for Neurobiology Researchers
EPEMED the European Personalised Medicine association appoints its General Delegate and several new board members















































