Phase 2 Study Confirm High Response Rate of Micromet’s Blinatumomab in Patients with Acute Lymphoblastic Leukemia
Micromet, Inc. announced data from its completed phase 2 clinical trial with blinatumomab in patients with B-precursor acute lymphoblastic leukemia (ALL). The data were presented at the 51st Annual Meeting of the American Society of hematology (ASH). Blinatumomab is a CD19-specific, T cell-engaging BiTE antibody designed to direct a patient’s own T cells against cancer cells inducing a self-destruction process in cancer cells.
A total of 21 patients were treated in a phase 2 clinical trial performed in collaboration with the German Multicenter Study Group on Adult Lymphoblastic Leukemia (GMALL). After having received extensive chemotherapy, all patients had ALL malignant cells persisting in their bone marrow, a disease state referred to as minimal residual disease (MRD). The primary endpoint of the clinical trial was the elimination of these cancer cells to an undetectable level in at least 22% of patients. 80% of the evaluable patients (16 of 20 patients) achieved the primary endpoint, all of them already during the first treatment cycle. The responses appear to be durable, with patients free of relapse for currently up to 15 months.
Overall, blinatumomab was well tolerated. The most common adverse events included lymphopenia, leucopenia, pyrexia and hypoimmunoglobulinemia. One patient had to discontinue treatment due to a fully reversible neurological adverse event, and was therefore not evaluable for response assessment.
“The data from this completed phase 2 study confirm the high response rate reported earlier this year from the ongoing study,” commented Dr. Jan Fagerberg, Micromet’s Chief Medical Officer. “We expect that the positive risk/benefit profile of blinatumomab in ALL will pave the way for a pivotal trial and a fast track to market in this indication.”
Most read news
Topics
Organizations
Other news from the department research and development

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Targeted synthesis of natural products with light - Potential pathway for drug development using photoreactions
Norwegian Team Completes Cod Genome
Ferriman-Gallwey_score
GE Healthcare completes acquisition of Whatman plc
Osmotic_demyelination_syndrome
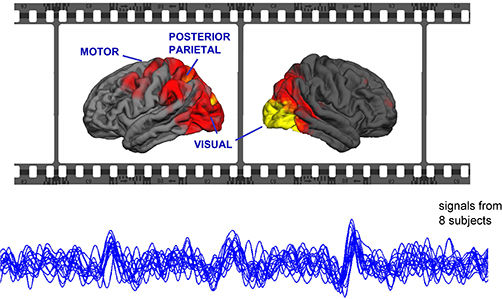
Movies synchronize brains - When we watch a movie, our brains react to it immediately in a way similar to other people's brains.
Innovative genetic and cellular techniques help identify multiple disease targets
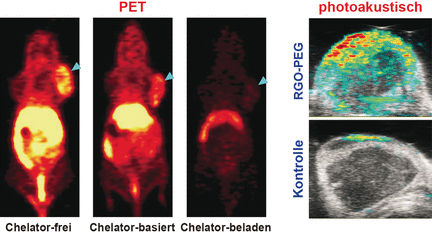
Direct radiolabeling of nanomaterials
Research will speed tracing bacteria behind salmonella outbreaks