Novartis completes shipment of US supply of Fluvirin seasonal influenza vaccine
Novartis announced that the company has completed its entire shipment of seasonal influenza vaccine to the United States for the 2009/2010 season. As previously anticipated, the company delivered 27 million doses of Fluvirin® influenza virus vaccine, which has been approved by the U.S. Food and Drug Administration (FDA). Novartis completed this season's shipment earlier than in previous years, in anticipation of demand for earlier vaccination with seasonal influenza vaccine created by the current global A(H1N1) influenza pandemic.
"Novartis is pleased to have delivered more seasonal influenza vaccine to the US market by the end of September than we have in any previous year allowing more people to get their vaccine early in the season," said Andrin Oswald, CEO of Novartis Vaccines and Diagnostics. "We are relieved to have been able to complete our deliveries ahead of schedule despite the challenging task to produce large quantities of A(H1N1) pandemic vaccines at the same time. We hope that the early delivery of our Fluvirin vaccine will help physicians and public health officials better prepare for the upcoming flu season and balance the needs for pandemic and seasonal vaccination."
On September 27, Novartis also began shipments of the first doses of its influenza A(H1N1) 2009 monovalent vaccine to the United States. The early shipment is the first of an accelerated effort to provide as much A(H1N1) vaccine as soon as possible, despite the low yield seen with the initial production seed strain provided by the World Health Organization. Production has switched to a new higher yielding seed strain which will allow deliveries of higher volumes later in the year.
Novartis Influenza A(H1N1) 2009 monovalent vaccine was approved by the FDA on September 15, 2009. The A(H1N1) vaccine is an inactivated subunit vaccine approved for active immunization of persons 4 years of age and older, including patients with underlying chronic medical conditions. The US Department of Health and Human Services (HHS) awarded Novartis two contracts totaling USD 979 million for purchase of H1N1 bulk vaccine and the Novartis proprietary MF59 adjuvant.
Most read news
Organizations
Other news from the department business & finance

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
See the theme worlds for related content
Last viewed contents
A Change of Culture for Healthcare
Medicyte GmbH Benefits From Multi-Million Euro EU Grant VascuBone
Pneumonoultramicroscopicsilicovolcanoconiosis
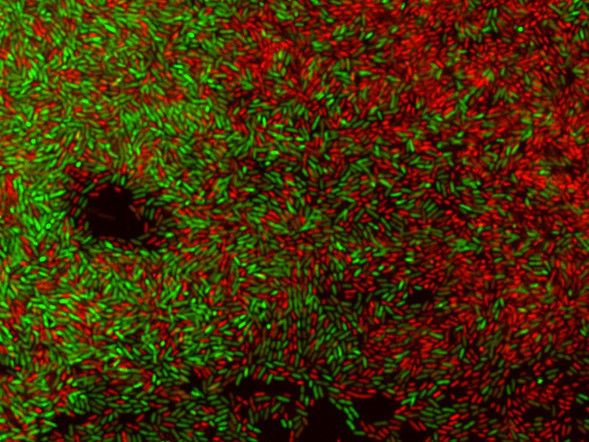
Biofilms - an invisible threat to food safety - Hotspots for biofilms

Snake venom treatment investigated as antibiotic alternative for eye infections