Enzyme inhibitor takes an unexpected approach toward blocking cancer-promoting protein
Advertisement
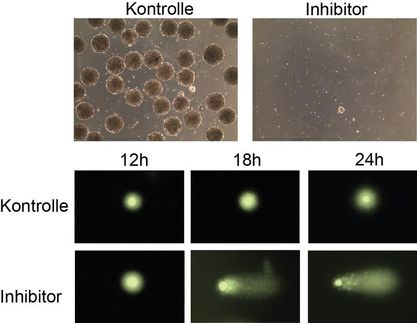
Scientists at Fox Chase Cancer Center have discovered a unique method of attack that may be used to inhibit signaling enzymes called kinases, which often have a role in sustaining drug-resistant cancerous cells. They have confirmed that IPA-3, a small molecular inhibitor of a kinase called PAK1, targets the enzyme's regulatory domain, mimicking how enzymes are naturally regulated within cells.
"Typically, research has focused on ways of blocking the active site of enzymes, the part of the enzyme that performs a particular task," says Jeffrey R. Peterson, Ph.D, an assistant professor Fox Chase's Cancer Genetics and Signaling program and co-author of the article. "The structure of active site, however, is often shared among kinases, which makes it tough to target a particular kinase without accidentally inhibiting a related enzyme."
"By targeting PAK1's specific regulatory domain, IPA-3 is highly selective molecule that takes a more-or-less backdoor approach to shutting down a kinase, " Peterson says. "If we can create drugs that take advantage of this mechanism, we could create new combination therapies that will allow doctors to kill what might otherwise be drug-resistant cells."
Peterson and Julien Viaud, a postdoctoral researcher in the Peterson lab, published their findings in the journal Molecular Cancer Therapeutics. The researchers previously identified IPA-3 from a screen of 33,000 candidates, and the molecule has since gone on to become an important subject of study by cancer laboratories around the world. In the article, the researchers use a variety of techniques to define how IPA-3 interacts with PAK1.
"We found definitive proof that IPA-3 fit into and binds to PAK1's autoregulatory domain, the part of the enzyme where it can, essentially, shut itself off when necessary," Peterson says. "Our tests also demonstrate that IPA-3 is highly selective for PAK1, which means that it is less likely it will also turn off other kinases unintentionally."
The idea is not entirely without precedent, the cancer drug Gleevec, for example, is unusually selective for its target by binding to a region outside of the active site that is less common among kinases. By defining the regulatory domain as a useful target for inhibition, however, researchers now have a specific place to look when trying to develop new therapeutics for protein regulation, Peterson says.
According to Peterson, small molecule inhibitors such as IPA-3, are promising tools for drug discovery. While IPA-3 itself might not be a suitable as a drug for use in humans, the molecule could form the conceptual basis of a new targeted therapeutic.