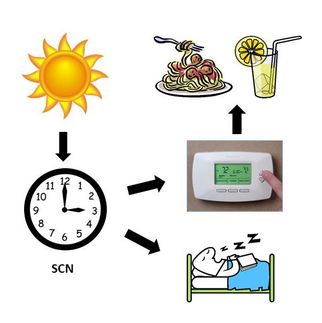
Central gears of the mammalian circadian clock exposed
The circadian clock, a 24-hour metabolic rhythm governing sleep cycles and other physiological processes, has long been known to play a central role in regulating the daily activities of living organisms. Its detailed biochemical mechanisms, however, have largely remained a mystery. That mystery is one step closer to being unraveled with the latest discovery by a research team led by Hiroki R. Ueda of the RIKEN Center for Developmental biology and Joseph S. Takahashi of Northwestern University, published in the Proceedings of the National Academy of Sciences. Researchers analyzed 1260 pharmacologically active compounds in mouse and human clock cell lines and identified 10 which exerted the greatest impact on the clock cycle. Surprisingly, all but one were found to target a single enzyme, the inhibition of which, researchers showed, dramatically extends this cycle from 24 hours to more than 48 hours. That the circadian clock may be regulated by relatively simple processes involving only a handful of molecules, a possibility indicated by this result, overturns conventional thinking on the topic. The more important finding that the inhibition process identified is insensitive to changes of as much as 10 degrees celcius further hints at a breakthrough in the related puzzle of temperature compensation: how circadian clocks maintain constant periodicity over a broad range of temperatures. Taken together, these findings suggest the need to fundamentally revise existing models of the mammalian circadian clock. They also point the way toward novel approaches to treatment of sleeping disorders and other debilitating clock-related conditions.
Most read news
Organizations
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
More news from our other portals
Last viewed contents
Generex Receives New US Patent for its Proprietary Buccal Drug Delivery Platform Technologies - Patent covers Method for Administration of Insulin to the Buccal Region