Princeton team learns why some drugs pack such a punch
Advertisement
By studying the intricate mechanisms at work in protein production, a Princeton-led team has discovered why certain kinds of antibiotics are so effective. In doing so, they also have discovered how one protein protects against cell death, shedding light on a natural cancer-fighting process.
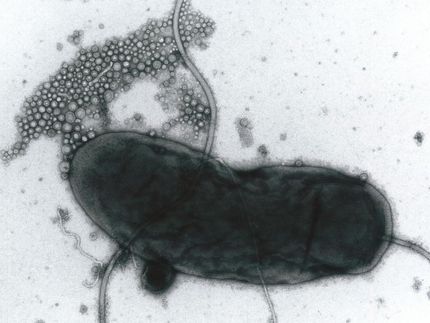
In a study appearing in Science , Thomas Silhavy, Princeton's Warner-Lambert Parke-Davis Professor of Molecular Biology, and Johna van Stelten, a graduate student, working with two Swiss researchers have uncovered how some antibiotics in common use for 50 years - tetracycline and chloramphenicol - can be so lethal against certain strains of bacteria. Simply put, these drugs plug things up.
Silhavy and van Stelten had been studying the mechanism by which proteins - from antibodies to hormones - are produced in bacteria's cytoplasm, the gooey substance that makes up the cell's interior, and then transported where they are needed. The spaghetti-like proteins exit the bacteria's cytoplasm through microscopic tubes known as translocators.
Sometimes, proteins fold up accidentally and jam the translocator. "Proteins go through the translocator, like a piece of spaghetti through a hole," Silhavy said. "But if you can imagine if you were to tie knots in the spaghetti, it wouldn't be able to get through; it gets stuck."
What happens then is ugly, according to Silhavy and van Stelten, who were the first ever to observe the event. The bacterial cell actually attacks the jammed translocator, decimating it. The researchers wondered what might happen in a more complex scenario, such as if antibiotics were introduced into the cell cytoplasm to purposely thwart bacteria.
The scientists found that the antibiotics tetracycline and chloramphenicol cause the ribosomes to stop midway through the process of making proteins, leaving partially constructed proteins stuck to the ribosome, jamming the translocator in the bacteria.
"This is very similar to plugging the translocator with a folded protein and, sure enough, this also causes translocator destruction," Silhavy said. "It's like putting an anchor on the spaghetti instead of a knot. They are stuck and dead forever."
Researchers had been confused as to why these antibiotics seemed to be so adept at killing some kinds of bacteria more quickly than others. These experiments provide an explanation. Translocators are essential for life and, if some bacteria have fewer translocators from the start, then they are more vulnerable to such an attack.
"While it has been known for many years that these antibiotics work by inhibiting bacterial protein synthesis, it was not clear why some bacteria in a population appeared more susceptible than others," van Stelten said. "Our work has identified a new reason why these antibiotics are lethal to bacteria that may help explain these earlier findings."