A potential drug for malaria?
Advertisement
An international collaboration of chemists have synthesised an o-quinodimethane that reacts with triplet oxygen to form a stable endoperoxide. o-quinodimethanes (o-xylylenes) have attracted great interest as they are useful building blocks for a variety of polycyclic compounds, that often exhibit biological activity. This has lead to increased activity from chemists interested in determining whether o-quinodimethanes are diradical or tetraene species.
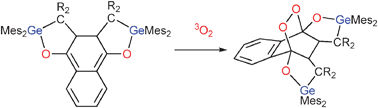
Heinz Gornitzka, Jean Escudié and colleagues from the University of Toulouse, France, in partnership with researchers at the Sidi Mohamed Ben Abdellah University, Morocco and collaborators at the University of California, have synthesised a stable substituted o-quinodimethane by a one-step procedure, from a germene and 1,4-naphthoquinone. Gornitzka and his team go on to show the great reactivity of the o-quinodimethane by reacting it with triplet oxygen to form an endoperoxide.
Gornitzka believes that this approach will lead to a generalisation of the reaction -changing the substituents on germanium and on the starting materials to determine the factors that affect the stabilisation of o-quinodimethane species. ‘We also plan to perform the synthesis of such derivatives from other doubly bonded compounds, for example >Si=C< to determine the influence of a heavier element on the stability and reactivity,’ he says.
Gornitzka hopes to probe the potential biological activity of the endoperoxide obtained from the reaction of the quinodimethane and oxygen, as most active drugs for the treatment of malaria present an endoperoxide structure. ‘We will extend the biological screening to other endoperoxides, to try to understand the relationship between structure and activity, in order to synthesise the most active compound,’ he adds.
Original publication: Ghereg et. al., Chem. Commun., 2009.