NTU professor discovers method to efficiently produce less toxic drugs using organic molecules
Nanyang Technological University (NTU)'s Associate Professor Zhong Guofu has made a significant contribution to the field of organic chemistry, in particular the study of using small organic molecules as catalysts, in the synthesis process called organocatalysis. Such synthesis process takes place for example, during the production of chiral drugs.
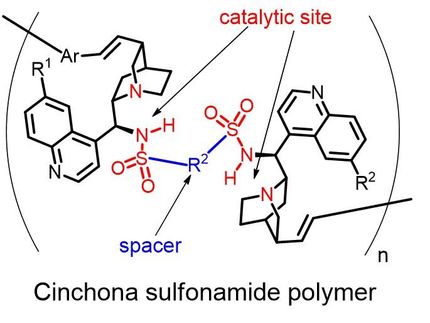
In his study, Professor Zhong, who is from NTU's School of Physical and Mathematical Sciences, has successfully created the first example where an organocatalyst is able to be 'recycled' (i.e. multiple reactions achieved with the recycled catalyst) during the synthesis process thus increasing its yield/effectiveness. Previously no one has been able to 'recycle' the organocatalysts directly (i.e. only single reactions performed) leading to the limitation of the use of organocatalysis in the industry.
This ability to 'recycle' and produce multiple reactions thus increases the efficacy of the organocatalysis, making it a more efficient process, something that has not been demonstrated before. It also means that fewer chemicals are used in the synthesis process, making it a far more 'green' and less toxic process.
Professor Zhong has written a paper on his discovery, which has been published in a recent edition of the scientific journal ChemComm.
The study of organocatalysis using organic molecules (which exists in nature, e.g. protein, amino acids) is a relatively new idea that started less than 10 years ago. The present dominant catalysts used in such synthesis process are 'ligand-metal catalysts' (such as ligand-copper, -palladium, -platinum, -ruthenium etc). However when compared to organocatalysts, ligand-metal catalysis is considered less 'green' and thus more 'toxic'.
However, the problem with using organocatalysts is that it is usually not an efficient or cost effective process since relatively a high catalyst loading is needed, compared to ligand-metal catalysis.
Professor Zhong is seeking patent in the United States for his hi process, which will be useful for the synthesis of certain chiral drug molecules which will be less toxic and produced under more efficient processes. The other advantage is that this process is considered 'highly enantioselective' – producing asymmetric synthesis that is desirable, for example, in synthesising certain drugs with chiral centers.
Professor Zhong is also filing for another patent related to his findings on domino synthesis, where the production process of one of the leading anti-cholesterol drugs in the world will be able to be shortened from its present 11 production steps to only 2-3 steps in the synthesis of its core intermediate. Pharmaceutical firms have expressed interest in adopting his methodology in their drug discovery and production process.
Topics
Organizations
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.