Proximagen Initiates Phase I Clinical Trial of PRX167700
Oral Vascular Adhesion Protein-1 antagonist for the treatment of inflammation in rheumatoid arthritis and psoriasis
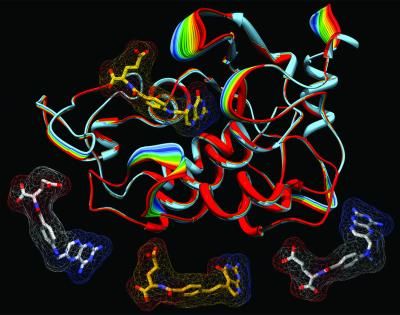
Proximagen Group plc announced that it had commenced dosing in a Phase I clinical trial of PRX167700, a Vascular Adhesion Protein-1 (VAP-1) antagonist, for the treatment of inflammation in rheumatoid arthritis (RA) and psoriasis.
Proximagen’s PRX167700 is an oral drug candidate that is expected to work by regulating the movement of immune cells from the blood into sites of inflammation, thereby modulating the underlying inflammatory process and relieving the symptoms of inflammation. This mode of action provides the potential for a disease modifying effect, whereby modulating the movement of the damaging immune cells to the sites of inflammation also stops further damage caused by the disease. In addition to efficacy shown in models of RA, PRX167700 has demonstrated efficacy in models of multiple sclerosis and inflammatory pain. Regulating the movement of immune cells is also important in these two diseases, indicating that PRX167700 has potential utility in multiple disease areas of high unmet medical need.
This Phase I trial is a randomised, double-blind, placebo-controlled study to assess the safety, tolerability and pharmacokinetics of single and multiple ascending oral doses of PRX167700 in healthy male subjects, and the effect of food on the pharmacokinetics of a single oral dose of the drug. The study will also investigate the relationship between the pharmacokinetic and pharmacodynamic effects of single and multiple ascending oral doses of PRX167700. The first results are anticipated in H2 2012.
PRX167700 was developed by Proximagen following the acquisition of Cambridge Biotechnology Limited in late 2009. In addition to PRX167700, Proximagen has two clinical stage CNS compounds in development for epilepsy and a pipeline of other clinical and preclinical assets in the fields of CNS, inflammation and oncology.
Most read news
Other news from the department research and development

Get the life science industry in your inbox
From now on, don't miss a thing: Our newsletter for biotechnology, pharma and life sciences brings you up to date every Tuesday and Thursday. The latest industry news, product highlights and innovations - compact and easy to understand in your inbox. Researched by us so you don't have to.